��Ŀ����
����Ŀ����1�����õ���̬��������ʳϰ�߿�����������������
�ٽ�����������Ӱ���˺ܶ��˵�������������γɵ���Ҫ��Ⱦ����_____������ĸ����
a��SO2 b��NO2 c��PM2.5
�������������������ڱ��������������й����������Ĵ�����������_____��
a���ó��������������� b���Ͼ�����¶����� c���Ͼɵ�ؾ͵�����
�������߲˸���ά����C���߲����Ա����ʱά����C����ʧС���ɴ��Ʋ�ά����C���ܾ��еĻ�ѧ������_____��
��2�����ϵIJ��Ϸ�չ���Դٽ���������
������Ʒ���������ԭ����_____��
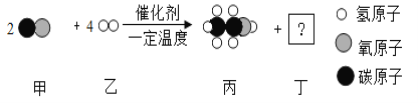
�ڵ����裨Si3N4����һ�������²��ϣ��ɸߴ���͵�����1300��ʱ��Ӧ�Ƶġ���ѧ����ʽΪ_____��
��3����ֹʳƷ���ʣ����ڰ�װ���з�һ���������
��д���������������ˮ�Ļ�ѧ����ʽ_____��
����ЩʳƷ�з���һС�����ۣ�������ʳƷ�в�������_____����_____���ã����һ�����_____����_____���á�
���𰸡�c a ���ȶ��Բ� ���ܺ�������Ӧ��������������Ĥ 3Si+2N2 ![]() Si3N4 CaO+H2O�TCa��OH��2 ˮ���� ���� ���� ��ֹʳƷ������
Si3N4 CaO+H2O�TCa��OH��2 ˮ���� ���� ���� ��ֹʳƷ������
��������
��1���ٽ�����������Ӱ���˺ܶ��˵�������������γɵ���Ҫ��Ⱦ����PM2.5��
���c��
��a���ó����������������������ڱ���������
b���Ͼ�����¶����ջ���Ⱦ������
c���Ͼɵ�ؾ͵��������Ⱦ������ˮ�塣
���a��
�������߲˸���ά����C���߲����Ա����ʱά����C����ʧС���ɴ��Ʋ�ά����C���ȶ��Բ
������ȶ��Բ
��2��������Ʒ���������ԭ�������ܺ�������Ӧ��������������Ĥ��
������ܺ�������Ӧ��������������Ĥ��
�ڵ����裨Si3N4����һ�������²��ϣ��ɸߴ���͵�����1300��ʱ��Ӧ�Ƶġ���ѧ����ʽΪ��3Si+2N2 ![]() Si3N4��
Si3N4��
���3Si+2N2 ![]() Si3N4��
Si3N4��
��3���������ƺ�ˮ��Ӧ�����������ƣ���Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O�TCa��OH��2��
���CaO+H2O�TCa��OH��2��
����ЩʳƷ�з���һС�����ۣ�������ʳƷ�в�������ˮ��������������ã����һ��������������ֹʳƷ���������á�
���ˮ�����������������ֹʳƷ��������

 �ִʾ��ƪϵ�д�
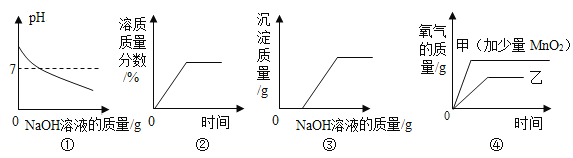
�ִʾ��ƪϵ�д�����Ŀ��С������H2O2��Һ��O2��ʵ��̽����������й��̣��ش��й����⡣
��1��MnO2����������5mL5%��H2O2��Һ�м�������MnO2�����������������ݡ�д����H2O2��Һ�Ʊ�O2�����ֱ���ʽ��________��
��2����5mL5%��H2O2��Һ�м���2��һ��Ũ�ȵ� FeCl3��Һ�����������������ݡ�
���������ϣ�FeCl3��Һ����Ҫ������������H2O��Fe3+��Cl-
��������⣩��������H2O2��Һ�ķֽ�������ã�
��������裩����һ��������H2O
�������������Fe3+
��������������_______��
���������ۣ�����һ�����ܳ�����������_______��
��ʵ��̽����
���� | ʵ����� | ʵ������ | ���� |
�� | �����������䣬��H2O2��Һ�м���NaCl��Һ | �����Ա仯 | Na+��Cl��û�д����� |
�� | �����������䣬��H2O2��Һ�м���Na2SO4��Һ | �����Ա仯 | __________ |
�� | �����������䣬��H2O2��Һ�м���Fe2(SO4)3��Һ | ���������������� | Fe3+�д����� |
��ʵ����ۣ�����_______������������һ����һ�ּ��費������
��֪ʶ��չ���Ƚϴ�������ѭ�����õĽǶȷ�����__���ѧʽ�����ʺ����÷�Ӧ�Ĵ�����