��Ŀ����
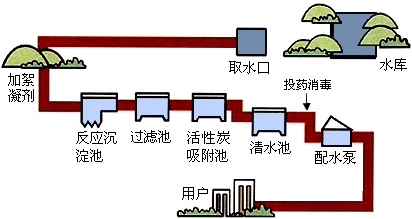
��6�֣���Ȼ���е�ˮ������������ԺͲ��������ʣ�ͨ������;������ʹˮ�õ���ͬ�̶ȵľ�������ͼ������ˮ������ˮ����ʾ��ͼ��
��1����������ˮ�ķ����У��������������������������������ȡ�
��2��Ͷҩ������Ŀ����ɱ��ˮ�е�ϸ���������ͼ����档����ˮ�����ö������ȣ�ClO2������������������Ԫ�صĻ��ϼ�Ϊ ��������й�ҵ�ƶ������ȵĻ�ѧ��Ӧ����ʽ��Cl2 + 2 NaClO2 ="=" 2 ClO2 + ��
��3������Ӧ����ϧÿһ��ˮ���������������ڽ�Լ��ˮ���� ��
| A��ϴ�˵�ˮ�������� | B��δ����Ŀ�Ȫˮ���ֵ��� |
| C���ò���ϵ���ˮ��ϴ��� | D��ϴ���ԡҺʱ������ˮ��ͷ |
��1������ ���� ��2��+4 2NaCl ��3��BC
��4��Ca(HCO3)2 CaCO3+H2O+CO2��
CaCO3+H2O+CO2��
����

��ϰ��ϵ�д�
�����Ŀ