��Ŀ����
�����ڸ�ϰ�����Ļ�ѧ����ʱ���֣���λ����ǰ��Ľ������û������ᡢϡ�����е��⡱��Ϊʲô������ϡ�����أ���������п��Ͷ��Ũ�����У������н϶�����ݷų������ŵ�һ�ɴ̼�����ζ��
[�������]���ɵ�������ʲô��
���������SO2��
���������H2��
���������SO2��H2�Ļ�����壮
[��������]
1������������һ����ɫ���д̼�����ζ���ж����壮������ˮ�Һ�ˮ������Ӧ�����ᣮ�ڳ��¡���ѹ�£�1���ˮ��Լ���ܽ�40����Ķ�������
2��SO2����ʹƷ����Һ��ɫ��
[����]���������ԭ����
[ʵ��̽��]�����������룬����������·�����
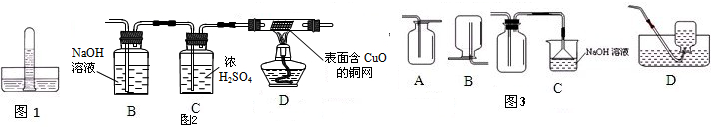
ʵ��һ���ռ�һ�Թ�п��Ũ���ᷴӦ���ɵ����壬�����ڵ�����ɫʯ����Һ��ˮ���У���ͼ1�������ݹ۲쵽��ʵ���������ɵ�������ȷ��SO2�Ĵ��ڣ����۲쵽��ʵ��������
ʵ������������������ͼ2��ʾ��ʵ��װ�ã�п����Ũ���ᷴӦ���ɵ�����ΪX���Ҹ�װ����ȥ�������ռ���������ͨ�룬��һ�����ȼD���ƾ��ƣ�һ��ʱ����ֱ��溬CuO��ͭ���ɺ�ɫ��Ϊ�����ĺ�ɫ���Թܿ���ˮ�����֣�
���ʵ��һ������֪����3������
[��������]
��1��װ��B��C�����÷ֱ���ʲô��
��2��D�������Ļ�ѧ��Ӧ����ʽ��
��3���ٴβ������ϣ�Ũ�������ǿ�����ԣ��������Ӧʱ��������������ѧ��Ӧ����ʽ
Ϊ��Zn+2H2SO4��Ũ���TZnSO4+SO2��+2H2O������ʵ���в���������ԭ����ʲô��
��4��ͼ3���ռ�SO2��װ�������������
��5����100��δ֪��������������������Һ�����ն����������壬����ȫ��Ӧ���������Һ������Ϊ112.8�ˣ�������������Һ�����������Ƕ��٣�

[�������]���ɵ�������ʲô��
���������SO2��
���������H2��
���������SO2��H2�Ļ�����壮
[��������]
1������������һ����ɫ���д̼�����ζ���ж����壮������ˮ�Һ�ˮ������Ӧ�����ᣮ�ڳ��¡���ѹ�£�1���ˮ��Լ���ܽ�40����Ķ�������
2��SO2����ʹƷ����Һ��ɫ��
[����]���������ԭ����
��������ɫ��ζ������
��������ɫ��ζ������
��[ʵ��̽��]�����������룬����������·�����
ʵ��һ���ռ�һ�Թ�п��Ũ���ᷴӦ���ɵ����壬�����ڵ�����ɫʯ����Һ��ˮ���У���ͼ1�������ݹ۲쵽��ʵ���������ɵ�������ȷ��SO2�Ĵ��ڣ����۲쵽��ʵ��������
�Թ��е�ˮ���������Թ��е�Һ���ɺ�ɫ
�Թ��е�ˮ���������Թ��е�Һ���ɺ�ɫ
��ʵ������������������ͼ2��ʾ��ʵ��װ�ã�п����Ũ���ᷴӦ���ɵ�����ΪX���Ҹ�װ����ȥ�������ռ���������ͨ�룬��һ�����ȼD���ƾ��ƣ�һ��ʱ����ֱ��溬CuO��ͭ���ɺ�ɫ��Ϊ�����ĺ�ɫ���Թܿ���ˮ�����֣�
���ʵ��һ������֪����3������
[��������]
��1��װ��B��C�����÷ֱ���ʲô��
��2��D�������Ļ�ѧ��Ӧ����ʽ��
H2+CuO
Cu+H2O
| ||
H2+CuO
Cu+H2O
�� ������һ�㲻Ҫ��
| ||
��3���ٴβ������ϣ�Ũ�������ǿ�����ԣ��������Ӧʱ��������������ѧ��Ӧ����ʽ
Ϊ��Zn+2H2SO4��Ũ���TZnSO4+SO2��+2H2O������ʵ���в���������ԭ����ʲô��
��4��ͼ3���ռ�SO2��װ�������������
C
C
��5����100��δ֪��������������������Һ�����ն����������壬����ȫ��Ӧ���������Һ������Ϊ112.8�ˣ�������������Һ�����������Ƕ��٣�

������[����]������������ɫ��ζ���������
[ʵ��̽��]���ݶ�������������ˮ�ҳ����ԣ��Լ�������ԭ����ͭ���������
[��������]��1������ÿ��װ���е�ҩƷ�����ʷ���������
��2�����ݷ���ʽ��дԭ����д
��3�����ݽ��������ᷴӦ���ص����
��4�����ݶ���������ܽ����Լ�������Ⱦ�����
��5�����ݻ�ѧ����ʽ��֪��������������������������Һ�����ʵ���������
[ʵ��̽��]���ݶ�������������ˮ�ҳ����ԣ��Լ�������ԭ����ͭ���������
[��������]��1������ÿ��װ���е�ҩƷ�����ʷ���������
��2�����ݷ���ʽ��дԭ����д
��3�����ݽ��������ᷴӦ���ص����
��4�����ݶ���������ܽ����Լ�������Ⱦ�����
��5�����ݻ�ѧ����ʽ��֪��������������������������Һ�����ʵ���������
����⣺[����]��Ϊ��������ɫ��ζ�����壬����Ŀ�в����������Ǵ̼�����ζ���壬�ʲ�������
[ʵ��̽��]��������������ˮ��������ˮ�����ԣ���ʹ��ɫʯ����Һ��죬�������Թ��е�ˮ���������Թ��е�Һ���ɺ�ɫ����ȷ��һ���ж���������Ϊ����ͨ��װ��D��CuO��ͭ���ɺ�ɫ��Ϊ�����ĺ�ɫ���Թܿ���ˮ�����֣�˵�������к���������
[��������]
��1���������������������Ʒ�Ӧ�����װ��B������������Һ��������SO2��װ��C�����������壨�����������е�ˮ������
��2��D����������ԭ����ͭ����ѧ����ʽΪH2+CuO
Cu+H2O
��3��������Ũ���ᷴӦ������������������Zn��ŨH2SO4��Ӧ�Ľ��У�ŨH2SO4Ũ����ϡ��Zn��ϡH2SO4��Ӧ�ɲ���H2
��4����Ϊ��������������ˮ����������ˮ���ռ��������������Ⱦ�����������ŷŵ������У��������ʵ��ռ�������C
��5���⣺������������Һ��������������Ϊx
2NaOH+SO2�TNa2SO3+H2O
80 64
100g��x 112.8g-100g
=
x=16%
������������Һ����������������16%
�ʴ�Ϊ��[����]��������ɫ��ζ�����壮
[ʵ��̽��]���Թ��е�ˮ���������Թ��е�Һ���ɺ�ɫ
[��������]
��1��װ��B������������Һ��������SO2��װ��C�����������壨�����������е�ˮ������
��2��H2+CuO
Cu+H2O
��3������Zn��ŨH2SO4��Ӧ�Ľ��У�ŨH2SO4Ũ����ϡ��Zn��ϡH2SO4��Ӧ�ɲ���H2
��4��C
��5��16%
[ʵ��̽��]��������������ˮ��������ˮ�����ԣ���ʹ��ɫʯ����Һ��죬�������Թ��е�ˮ���������Թ��е�Һ���ɺ�ɫ����ȷ��һ���ж���������Ϊ����ͨ��װ��D��CuO��ͭ���ɺ�ɫ��Ϊ�����ĺ�ɫ���Թܿ���ˮ�����֣�˵�������к���������
[��������]
��1���������������������Ʒ�Ӧ�����װ��B������������Һ��������SO2��װ��C�����������壨�����������е�ˮ������
��2��D����������ԭ����ͭ����ѧ����ʽΪH2+CuO
| ||
��3��������Ũ���ᷴӦ������������������Zn��ŨH2SO4��Ӧ�Ľ��У�ŨH2SO4Ũ����ϡ��Zn��ϡH2SO4��Ӧ�ɲ���H2
��4����Ϊ��������������ˮ����������ˮ���ռ��������������Ⱦ�����������ŷŵ������У��������ʵ��ռ�������C
��5���⣺������������Һ��������������Ϊx
2NaOH+SO2�TNa2SO3+H2O
80 64
100g��x 112.8g-100g
| 80 |
| 100g��x |
| 64 |
| 12.8g |
x=16%
������������Һ����������������16%
�ʴ�Ϊ��[����]��������ɫ��ζ�����壮
[ʵ��̽��]���Թ��е�ˮ���������Թ��е�Һ���ɺ�ɫ
[��������]
��1��װ��B������������Һ��������SO2��װ��C�����������壨�����������е�ˮ������
��2��H2+CuO
| ||
��3������Zn��ŨH2SO4��Ӧ�Ľ��У�ŨH2SO4Ũ����ϡ��Zn��ϡH2SO4��Ӧ�ɲ���H2
��4��C
��5��16%
�����������ۺϿ����˽����Ļ�ѧ���ʡ�ʹ��������ע�������Լ���IJ������ʣ���������ļ�����Ҫ��������������̼�������������������ȣ�ѡ�����ʱ��Ҫ�����������ʵIJ��죮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ