��Ŀ����
��1��ijԪ��M���������У�MԪ������Ԫ�ص�������Ϊ21��8�������ԭ��������Ϊ7��2�����������Ļ�ѧʽΪ��2��һ���ܱ������У�����m��CO���Ӻ�n��O2���ӣ���һ�������³�ַ�Ӧ��������̼ԭ������ԭ�Ӹ�����Ϊ
��3�����Ȼ�����Һ�еμ�ϡ������Һ��ֱ���������ɳ��������˺���Һ����ǡ�õ����Ȼ�����Һ�������������ϡ������Һ�����ʵ���������Ϊ
��4��ͭ�ۺ�̿�۵Ļ�����ڿ����г�ַ�Ӧ�����ɵĺ�ɫ������ԭ������������ȣ���ԭ�������ͭ����������
��5���������⣨H2O2���������������ֽ�Ϊˮ������������3%�Ĺ���������Һ����Ԫ�ص���������Ϊ11.0%��������ȫ�ֽ⣬��Ԫ�ص������������ӵ�ֵΪ
��2����Ӧǰ��ԭ�Ӹ����Dz���ģ���ֱ������CO��O2�ķ��Ӹ���������ԭ�Ӹ���֮�ȣ�
��3��������Һ����ǡ�õ����Ȼ�����Һ�������������ᱵ����������ϡ������Һ���������������
��4������ͭ��Ӧ��������ͭ��ɫ���壬Cȼ���������壬���������ɵĺ�ɫ������ԭ������������ȣ���֪����ͭ����Ԫ����������C������������ͭ������������
��5�����ݹ�������ֽⷴӦ��֪����������ˮ������Һ��HԪ�ص��������䣬����Һ�����ڼ�С�������Ӧ����Һ�������ɼ�����Ԫ�ص������������ӵ�ֵ��
| 7 |
| 2 |
��Ϊ�����軯ѧʽΪFexOy����MԪ������Ԫ�ص�������Ϊ21��8����56��x��16��y=21��8�����x��y=3��4������ѧʽΪFe3O4���ʴ�Ϊ��Fe3O4��
��2����Ӧǰ��ԭ���غ㣬���ܱ�������m��CO���Ӻ�n��O2������̼ԭ������ԭ�Ӹ�����Ϊm����m+2n����
�ʴ�Ϊ��m����m+2n����
��3�����ݻ�ѧ��Ӧ����ʽ��֪������Һ����ǡ�õ����Ȼ�����Һ������ʱ�������ɵ����ᱵ����������������Һ��������
�������ᱵ������Ϊ233g������������Һ����������Ϊxg��
BaCl2+H2SO4�TBaSO4��+2HCl
98 233
x 233g
| 98 |
| x |
| 233 |
| 233g |
��������Һ����������Ϊ
| 98g |
| 233g |
��4����ͭ��Ӧ��������ͭ��ɫ���壬Cȼ���������壬�����ɵĺ�ɫ������ԭ������������ȣ�
������ͭ����Ԫ����������C����������ԭ�������̼������������������ͭ����Ԫ�ص�����������
ԭ�������̼����������Ϊ
| 16 |
| 80 |
��5������Һ����Ϊmg��������������Ϊxg����
2H2O2
| ||
68 32
m��3% x
| 68 |
| 32 |
| m��3% |
| x |
��Ӧ����Һ����Ϊm-0.014m=0.986mg��
��Ӧ��HԪ�ص���������Ϊ
| mg��11.0% |
| 0.986mg |
����Ԫ�ص������������ӵ�ֵΪ11.2%-11.0%=0.2%���ʴ�Ϊ��0.2%��

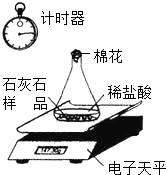 ij�о���С���������µ�ʵ�飺ȡ50gʯ��ʯ��Ʒ�����ʲ�����ˮ����������ѧ��Ӧ��Ҳ������Ԫ�أ�������ƿ�У�����������ϡ���ᣮʵ��װ������ͼ��ʾ����֪��ƿ��ͬƿ��ʯ��ʯ��Ʒ��ϡ�����Լ�ƿ������ʼʱ��������Ϊ300.0g��ʵ�����ݼ�¼���±���
ij�о���С���������µ�ʵ�飺ȡ50gʯ��ʯ��Ʒ�����ʲ�����ˮ����������ѧ��Ӧ��Ҳ������Ԫ�أ�������ƿ�У�����������ϡ���ᣮʵ��װ������ͼ��ʾ����֪��ƿ��ͬƿ��ʯ��ʯ��Ʒ��ϡ�����Լ�ƿ������ʼʱ��������Ϊ300.0g��ʵ�����ݼ�¼���±���| ʱ��/min | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 |
| ������ƽʾ��/g | 294.5 | 290.0 | 286.0 | 284.0 | 283.0 | 282.4 | M | 282.4 |
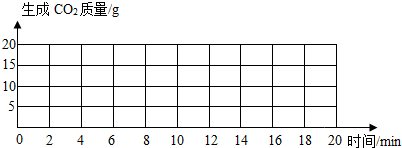
��2������ʵ�����ݼ�¼��������CO2��������ʱ���ϵ��ͼ��
��3��������ʯ��ʯ��Ʒ��̼��Ƶ���������������д��������̣�
��4��ȡ��ʯ��ʯ��Ʒ100g����������һ��ʱ�����ȴ�����ʣ������к���Ԫ�ص���������Ϊ41%���������������Ƶ�����������д��������̣�
��13�֣���ѧ������ѧϰ�ͽ�����ѧ֪ʶ��ͨ�����ԣ���ȷ���ر��ﻯѧ��Ϣ
��1�����û�ѧ������д��
��2����ԭ���� �� ��m�������������� �� ��2��ˮ������ ��
��̼����_______ ������þ��þԪ�صĻ��ϼ�Ϊ+2���� ��
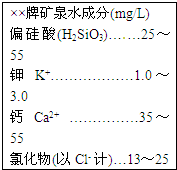
��2����ͼΪij��Ȫˮ��ǩ�IJ������ݣ�����ͼ����Ϣ�ش�
| �����ƿ�Ȫˮ�ɷ�(mg/L) ƫ����(H2SiO3)����25��55 ��K+������������1.0��3.0 ��Ca2+ ����������35��55 �Ȼ���(��Cl- ��)��13��25 |
��ƫ�����й�Ԫ�صĻ��ϼ�Ϊ�� ��
�ۿ�Ȫˮ�������Ȼ��ƵĻ�ѧʽΪ�� ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ
������������ȼ���� ��
���д����������ɵĻ��Ϸ�Ӧ�� ��
��������غͶ������̻���������� ��
��ľ̿ȼ���� ��
��13�֣���ѧ������ѧϰ�ͽ�����ѧ֪ʶ��ͨ�����ԣ���ȷ���ر��ﻯѧ��Ϣ
��1�����û�ѧ������д��
��2����ԭ���� �� ��m�������������� �� ��2��ˮ������ ��
��̼����_____ __ ������þ��þԪ�صĻ��ϼ�Ϊ+2���� ��
��2����ͼΪij��Ȫˮ��ǩ�IJ������ݣ�����ͼ����Ϣ�ش�
|
�����ƿ�Ȫˮ�ɷ�(mg/L) ƫ����(H2SiO3)����25��55 ��K+������������1.0��3.0 ��Ca2+ ����������35��55 �Ȼ���(��Cl- ��)��13��25 |
��ƫ�������� ����Ԫ����ɣ�ƫ����������⡢�衢��ԭ�ӵĸ�����Ϊ�� ��
��ƫ�����й�Ԫ�صĻ��ϼ�Ϊ�� ��
�ۿ�Ȫˮ�������Ȼ��ƵĻ�ѧʽΪ�� ��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ
������������ȼ���� ��
���д����������ɵĻ��Ϸ�Ӧ�� ��
��������غͶ������̻���������� ��
��ľ̿ȼ���� ��
 ��ѧ������ѧϰ�ͽ�����ѧ֪ʶ��ͨ�����ԣ���ȷ���ر��ﻯѧ��Ϣ
��ѧ������ѧϰ�ͽ�����ѧ֪ʶ��ͨ�����ԣ���ȷ���ر��ﻯѧ��Ϣ