��Ŀ����
(9��)����A ~ F�dz��л�ѧ�е�����ʵ�飬�밴Ҫ����գ�

(1)Cʵ���н����Ŀ���� ��
(2)Bʵ���к���ȼ�յĻ�ѧ����ʽΪ ��ʵ��˵�����������Լռ������ ��ʵ��ɹ��Ĺؼ��� (�����)��
��װ�������Ժã���ʵ��ǰ�н�ֹˮ�У��ۺ�������������
����ȴ���ٴ�ֹˮ�У���Ҫѡ�ý����ڵĿ�����
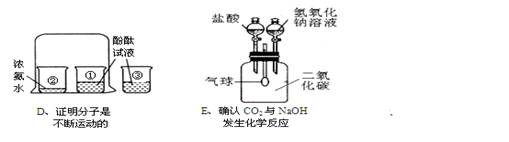
(3)Eʵ�� �ȵ�����������Һ����ʱ�۲쵽�������� ��������Ӧ�Ļ�ѧ����ʽΪ �ٵ�����ʱ�۲쵽�������� ��������Ӧ�Ļ�ѧ����ʽΪ
(4)����ʵ�����ܴﵽʵ��Ŀ������ȷ���� (����ĸ)��
(1)ɢ��(ʹ����������Ѹ����ɢ)�����(1��)
(2)4P + 5O2 ��ȼ 2P2O5 (1��)�� 1/5 (1��)�٢ڢۢ�(ȫ��1�֣������֡�)
(3)�ٵ�������������Һ���������ͣ�CO2 + 2NaOH == Na2CO3 + H2O(�ڵ�������������ݣ���������СNa2CO3 + 2HCl == 2NaCl + H2O + CO2��
(4)B��D��E
����������

 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�