��Ŀ����
�ܱ�������װ��ij������壬�õ��ȼ��ǡ����ȫ��Ӧ����֪�˻�����������H2��CO��O2�е����ֻ����������϶��ɣ���1�������������ֻ����H2��O2�������壬������H2 ������O2���ӵĸ���֮��Ϊ______��
��2�������������ֻ����CO��O2�������壬������CO������O2���ӵĸ���֮��Ϊ______��
��3������������к���H2��CO��O2�������壬��������Ϊ62g����������������Ϊ32g����H2��CO��O2���ߵ�������Ϊ______��
��4������������к���H2��CO��O2�������壬������֮��Ӧ�����������______��
���𰸡���������1������������������Ӧ�ķ�Ӧ����ʽ�����ɵó����Ӹ����ȣ�
��2������һ����̼��������Ӧ�ķ�Ӧ����ʽ�����ɵó����Ӹ����ȣ�
��3������������������Ӧ�Ļ�ѧ��Ӧʽ��������һ����̼�Ļ�ѧ��Ӧʽ�����������һ����̼��������Ȼ��д����ֵ���ɣ�
��4���������ǡ����ȫ��Ӧ��Ȼ�����һ����̼�������������ķ�Ӧ��ѧʽ��ȷ�����ǵ������������
����⣺��1�����������������Ļ�ѧ��Ӧʽ��2H2+O2 2H2O����֪������������Ӹ���֮��Ϊ2��1��
2H2O����֪������������Ӹ���֮��Ϊ2��1��
��2������һ����̼�������Ļ�ѧ��Ӧʽ��2CO+O2 2CO2����֪һ����̼�������Ӹ���֮��Ϊ2��1��
2CO2����֪һ����̼�������Ӹ���֮��Ϊ2��1��
��3����������Ϊ32g�������������Ϊ62g����������һ����̼��������=62g-32g=30g��
�裺������������xg����һ����̼�������ǣ�30-x��g
����2H2+O2 2H2O
2H2O
4 32
x 8x
2CO+O2 2CO2
2CO2
2��12+16��32
30-x
����8x+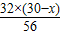 =32
=32
��֮�ã�x=2g
��CO������=30-2=28g
����������һ����̼����������������2��28��32����1��14��16��
��4���������������ϣ����ݻ�ѧ��Ӧʽ��2H2+O2 2H2O��2CO+O2
2H2O��2CO+O2 2CO2��CO��H2������������
2CO2��CO��H2������������
��ȣ���CO��H2�ķ���������O2������֮��Ϊ2��1��
�ʴ�Ϊ��
��1��2��1
��2��2��1
��3��1��14��16
��4��CO��H2��������������ȣ���CO��H2�ķ���������O2������֮��Ϊ2��1��
���������⿼���뻯ѧ��Ӧʽ��صļ��㡢�ƶϣ������Ѷȴ����Ŀ����Ҫͬѧ��ϸ�ģ��ſ����ã�
��2������һ����̼��������Ӧ�ķ�Ӧ����ʽ�����ɵó����Ӹ����ȣ�
��3������������������Ӧ�Ļ�ѧ��Ӧʽ��������һ����̼�Ļ�ѧ��Ӧʽ�����������һ����̼��������Ȼ��д����ֵ���ɣ�
��4���������ǡ����ȫ��Ӧ��Ȼ�����һ����̼�������������ķ�Ӧ��ѧʽ��ȷ�����ǵ������������
����⣺��1�����������������Ļ�ѧ��Ӧʽ��2H2+O2
 2H2O����֪������������Ӹ���֮��Ϊ2��1��
2H2O����֪������������Ӹ���֮��Ϊ2��1����2������һ����̼�������Ļ�ѧ��Ӧʽ��2CO+O2
 2CO2����֪һ����̼�������Ӹ���֮��Ϊ2��1��
2CO2����֪һ����̼�������Ӹ���֮��Ϊ2��1����3����������Ϊ32g�������������Ϊ62g����������һ����̼��������=62g-32g=30g��
�裺������������xg����һ����̼�������ǣ�30-x��g
����2H2+O2
 2H2O
2H2O 4 32
x 8x
2CO+O2
 2CO2
2CO22��12+16��32
30-x

����8x+
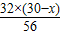 =32
=32��֮�ã�x=2g
��CO������=30-2=28g
����������һ����̼����������������2��28��32����1��14��16��
��4���������������ϣ����ݻ�ѧ��Ӧʽ��2H2+O2
 2H2O��2CO+O2
2H2O��2CO+O2 2CO2��CO��H2������������
2CO2��CO��H2��������������ȣ���CO��H2�ķ���������O2������֮��Ϊ2��1��
�ʴ�Ϊ��
��1��2��1
��2��2��1
��3��1��14��16
��4��CO��H2��������������ȣ���CO��H2�ķ���������O2������֮��Ϊ2��1��
���������⿼���뻯ѧ��Ӧʽ��صļ��㡢�ƶϣ������Ѷȴ����Ŀ����Ҫͬѧ��ϸ�ģ��ſ����ã�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ