��Ŀ����
��ľ����ũ�峣�õ�һ�ַʶ�������Ч�ɷ���K2CO3����ij��ȤС��Ϊ�˲ⶨ��ľ����K2CO3�ĺ�����ȡ���еIJ�ľ��100g����μ���ϡ������ǡ����ȫ��Ӧ������ϡ����50g�����˳�ȥ���ʣ��õ���Һ50.94g���������ľ���г�K2CO3�⣬�������ʾ�������ˮ��Ҳ�������ᷴӦ�����Լ��㣺�ٸò�ľ����K2CO3������������
�ڷ�Ӧ��������Һ�����ʵ�����������
���𰸡��������������ľ����K2CO3������Ϊx��������Ŀ�еķ�Ӧ�������ͷ�Ӧ���������ݻ�ѧ����ʽ����д����������д����ľ����ϡ���ᷴӦ�Ļ�ѧ����ʽ�����ݻ�ѧ����ʽ�ҳ�̼������Ȼ��ء�������̼�������ȣ���̼��ص������ɼ��������x��ʾ�������Ȼ��ء�������̼��������Ȼ����������г�����x�ķ��̣��ⷽ�����̼��ص��������Ӷ�����Ȼ��ص���������������������ļ��㹫ʽ����ٸò�ľ����K2CO3�������������ڷ�Ӧ��������Һ�����ʵ������������н��
����⣺�����ľ����K2CO3������Ϊx�������Ȼ��ص�����Ϊy�����ɶ�����̼������Ϊz��
K2CO3+2HCl=2KCl+H2O+CO2��
138 149 44
x y z

y=

z=
�������50g+x-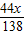 =50.94g
=50.94g
x=1.38g
���Բ�ľ����K2CO3������������ =1.38%
=1.38%
������KCl�������� =1.49g
=1.49g
��Ӧ��������Һ��������������Ϊ��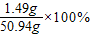 =2.9%
=2.9%
�𣺢ٸò�ľ����K2CO3������������1.38%��
�ڷ�Ӧ��������Һ�����ʵ�����������2.9%��
���������⿼����ݻ�ѧ����ʽ�Ļ������㣬���п��ؿ����ͣ��ѶȲ���Ҫ��ѧ������淶��ϸ�ģ�
����⣺�����ľ����K2CO3������Ϊx�������Ȼ��ص�����Ϊy�����ɶ�����̼������Ϊz��
K2CO3+2HCl=2KCl+H2O+CO2��
138 149 44
x y z

y=


z=

�������50g+x-
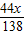 =50.94g
=50.94gx=1.38g
���Բ�ľ����K2CO3������������
 =1.38%
=1.38%������KCl��������
 =1.49g
=1.49g��Ӧ��������Һ��������������Ϊ��
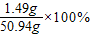 =2.9%
=2.9%�𣺢ٸò�ľ����K2CO3������������1.38%��
�ڷ�Ӧ��������Һ�����ʵ�����������2.9%��
���������⿼����ݻ�ѧ����ʽ�Ļ������㣬���п��ؿ����ͣ��ѶȲ���Ҫ��ѧ������淶��ϸ�ģ�

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ