��Ŀ����
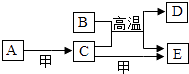
����Ŀ����ͼ�е�A��K�ֱ�������л�ѧ�еij������ʣ���֪��A��C���������Ԫ����ͬ�� G��ҺΪij���ʵ�ϡ��Һ��G��Ũ��Һ��ʹֽƬ��ڣ�ͼ�в���������δ����� 
��ش��������⣺
��1��д�����з�Ӧ�Ļ�ѧ����ʽ��
��A ![]() C+D����
C+D����
��F+H��E+C����
��2��ij��ѧ����С�齫E��ĩ����ϡG��Һ�У����Ȳ����Ϲ��뵥������D������I��Һ��д�����÷�Ӧ�Ļ�ѧ����ʽ ��
��3����ʵ���ҵ��Cʱ����C�м�������Na2SO4��H2SO4�������� ��
���𰸡�
��1��2H2O2 ![]() 2H2O+O2��,H2+CuO
2H2O+O2��,H2+CuO ![]() Cu+H2O
Cu+H2O
��2��2Cu+2H2SO4+O2 ![]() 2CuSO4+2H2O
2CuSO4+2H2O
��3����ǿˮ�ĵ�����
���������⣺A��Һ�м����ɫ��ĩB�����ɵ�C�ܵ�����ɵ�������D��F��˵��CΪˮ��A��C���������Ԫ����ͬ��˵��AΪ�������⣬��ɫ��ĩBΪ�������̣���������D�������������E���ȷ�Ӧ���ɺ�ɫ����H��˵��D����������FΪ������G��Ũ��Һ��ʹСľ����ڣ�˵��GΪ���ᣬ��ɫ����H�����ᷴӦ�õ���ɫ��ҺI��˵��IΪ����ͭ����HΪ����ͭ��EΪͭ����ˣ�
��1��A ![]() C+DΪ˫��ˮ�ķֽ⣬���������ڴ����������̵�������������ˮ���������ʷ�Ӧ�ķ���ʽΪ��2H2O2
C+DΪ˫��ˮ�ķֽ⣬���������ڴ����������̵�������������ˮ���������ʷ�Ӧ�ķ���ʽΪ��2H2O2 ![]() 2H2O+O2����
2H2O+O2����
F+H��E+C������������ͭ��ԭΪͭ�ķ�Ӧ���ʷ�Ӧ�ķ���ʽΪ��H2+CuO ![]() Cu+H2O��
Cu+H2O��
��2����E��ĩ����ϡG��Һ�У����Ȳ����Ϲ��뵥������D������I��Һ����ͭ��ϡ�����ڼ���ʱ��������Ӧ��������ͭ��Һ����������غ㶨�ɣ����ﻹӦ����ˮ���ʷ�Ӧ�ķ���ʽΪ��2Cu+2H2SO4+O2 ![]() 2CuSO4+2H2O��
2CuSO4+2H2O��
��3��ˮ�ĵ�����������ˮ�м�������Na2SO4��H2SO4��������ǿˮ�ĵ����ԣ����Դ��ǣ���ǿˮ�ĵ����ԣ�
�����㾫����������Ŀ����֪������������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�����֪ʶ���Եõ�����Ĵ𰸣���Ҫ����ע�⣺a����ƽ b������ c�����ţ�