��Ŀ����
 ����Fe��Fe2O3�Ĺ����������ͬѧΪ�˷����������Fe��Fe2O3�ĺ��������������ʵ�鷽����
����Fe��Fe2O3�Ĺ����������ͬѧΪ�˷����������Fe��Fe2O3�ĺ��������������ʵ�鷽����
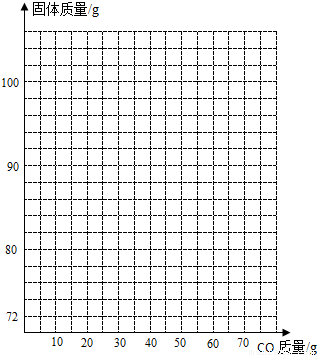
��ʵ�����ݡ�ʵ�鹲��¼������ʵ�����ݣ��ڢ��飬������ȫ���պ�ʯ��ˮ��������66g���ڢ��飬��ȫ��Ӧ����ȴ�����ʣ����������ΪWg��
����ʵ����Ƽ��й����ݽ��з��������
��1����������������� g��
��2���������Fe���ʵ���������Ϊ���٣���д��������̣�
��3���ڢ�������W�� g��д������������
��4������ͬѧ������ñ�������������Һȡ������ʯ��ˮ�����յڢ������������Ч�����ã����������ô˵���������� ���� ��
��1��66��1�֣�
��2������ѧ����ʽ2�֣�������4�֣�
�⣺����������Fe2O3������ΪX
|
Fe2O3+3CO==== 2Fe +3CO2
 160 132 2��
160 132 2��
X 66g
160:X = 132:66g
��� x= 80g��1�֣�
Fe���ʵ�������������100g – 80g��/100g ��100% = 20%��1�֣�
�𣺻�����������ʵ�����������20%��
��3��76��2�֣�
��4����Ϊ������������ˮ��������ͬ�����ı��͵�����������Һ��ʯ��ˮ�����ո���Ķ�����̼����2�֣�

 ��У����ϵ�д�
��У����ϵ�д�


 2Fe + 3CO2
2Fe + 3CO2

 2Fe+3CO2
2Fe+3CO2