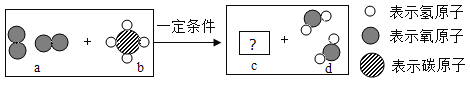
��Ŀ����
�⻯�ƣ�CaH2����һ�ְ�ɫϸ����״���壬����ˮ�������ҷ�Ӧ��������ð�����䷴Ӧ�Ļ�ѧ����ʽΪ��CaH2+2H2O= Ca(OH)2+_______���벹������������������ɽ�˶�Ա����Դ�ṩ����ij��ѧʵ��С��Ϊ̽���⻯�Ƶ����ʣ���һ������CaH2���뵽̼������Һ�У����������������ݣ�����Һ����ǡ���ַ�Ӧ����ˣ��õ���������Һ��������һ���е�������_________�������ƣ���Ϊ��һ��ȷ����Һ���������ʵijɷ֣����ǽ���������ʵ��̽����
��������룩����һ��NaOH �������NaOH��Ca(OH)2 ��������NaOH��Na2CO3�������ģ�NaOH��Na2CO3��Ca(OH)2 �������ۣ����һ����Ϊ�����IJ������������ǣ��û�ѧ����ʽ��ʾ��_______��
��ʵ����֤��
ʵ�� | ���� | ���� |
��1��ȡ������Һ�������е�������̼������Һ | ���������� | ����____������ |
��2����ȡ������Һ�������м����___________________ | ��_____________ | ���������� |
����˼����չ��
��1������NH4Cl����Һ�м���һ������CaH2����ַ�Ӧ��������������_______________��
��2����NaH�Ļ�ѧ������CaH2���ƣ���NaH��ˮ��Ӧ�Ļ�ѧ����ʽΪ_________________��
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д����ܱ������У��Ҵ����ȼ�գ��䷴Ӧǰ������ʵ������仯���±���ʾ����������ȷ����
���� | �� | �� | �� | �� |
��Ӧǰ��������g�� | 23 | 0 | 48 | m |
��Ӧ���������g�� | 0 | 27 | 0 | 45 |
A.�״������Ƿ�Ӧ���Ҵ� B.m=1
C.�÷�Ӧ���������ֻ�����Ӧ���� D.���붡�Ļ�ѧ������֮��Ϊ2:3


 B
B  C
C  D
D  E
E 
 ��O2��ҩƿ
��O2��ҩƿ ����װ��
����װ�� �ⶨ����O2�����
�ⶨ����O2����� ��֤O2�Ŀ�ȼ��
��֤O2�Ŀ�ȼ��