��Ŀ����
��֪���ֽⷴӦ2CH3COOH+Na2CO3=2CH3COONa+H2O+CO2���ɽ��У��ڳ����£������ͬŨ�ȵ�����������Һ��pH��| ���� | NaHCO3 | CH3COONa | NaClO | NaCN | Na2CO3 |
| pH | 8.6 | 8.8 | 10.3 | 11.1 | 11.6 |
A��CO2+H2O+2NaClO=Na2CO3+2HClO
B��CO2+H2O+NaClO=NaHCO3+HClO
C��CH3COOH+NaCN=CH3COONa+HCN
D��NaClO+CH3COOH=HClO+CH3COONa
���𰸡�������������Һ��pH����7�Լ��ԣ���pHԽ�����Խǿ���н��
����⣺A���ɱ���֪NaClO��Һ��pH=10.3��Na2CO3��Һ��pH=11.6������CO2+H2O+2NaClO=Na2CO3+2HClO���ܳ�������A����
B���ɱ���֪NaClO��Һ��pH=10.3��NaHCO3��Һ��pH=8.6������CO2+H2O+NaClO=NaHCO3+HClO�ܳ�������B��ȷ��
C���ɱ���֪NaCN��Һ��pH=11.1��CH3COONa��Һ��pH=8.8������CH3COOH+NaCN=CH3COONa+HCN�ܳ�������C��ȷ��
D���ɱ���֪NaClO��Һ��pH=10.3��CH3COONa��Һ��pH=8.8������NaClO+CH3COOH=HClO+CH3COONa�ܳ�������D��ȷ��
��ѡ��A��
�����������ǶԸ��ֽⷴӦ�Ŀ��飬������ص����˽���Һ�������pH�Ĺ�ϵ����������֪ʶ�����⣮
����⣺A���ɱ���֪NaClO��Һ��pH=10.3��Na2CO3��Һ��pH=11.6������CO2+H2O+2NaClO=Na2CO3+2HClO���ܳ�������A����
B���ɱ���֪NaClO��Һ��pH=10.3��NaHCO3��Һ��pH=8.6������CO2+H2O+NaClO=NaHCO3+HClO�ܳ�������B��ȷ��
C���ɱ���֪NaCN��Һ��pH=11.1��CH3COONa��Һ��pH=8.8������CH3COOH+NaCN=CH3COONa+HCN�ܳ�������C��ȷ��
D���ɱ���֪NaClO��Һ��pH=10.3��CH3COONa��Һ��pH=8.8������NaClO+CH3COOH=HClO+CH3COONa�ܳ�������D��ȷ��
��ѡ��A��
�����������ǶԸ��ֽⷴӦ�Ŀ��飬������ص����˽���Һ�������pH�Ĺ�ϵ����������֪ʶ�����⣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
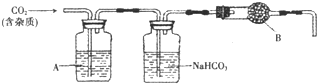
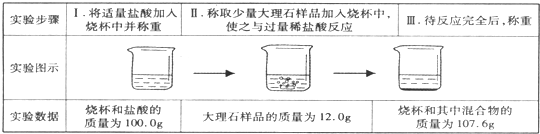
 CaCO3�㷺��������Ȼ�磬��һ����Ҫ�Ļ���ԭ�ϣ�ij�ִ���ʯ����Ҫ�ɷ�ΪCaCO3�⣬���������������С���С��ͬѧ�����ִ���ʯ��ϡ���ᷴӦ���ֱ�չ����̽�����������̽�����ش�������⣮
CaCO3�㷺��������Ȼ�磬��һ����Ҫ�Ļ���ԭ�ϣ�ij�ִ���ʯ����Ҫ�ɷ�ΪCaCO3�⣬���������������С���С��ͬѧ�����ִ���ʯ��ϡ���ᷴӦ���ֱ�չ����̽�����������̽�����ش�������⣮