��Ŀ����
ij��ѧ��ȤС��ͬѧ�����˼������ʯ��ˮ������������Һ��ʵ��̽��������һͬ����̽����
��������⣩��μ�����������ɫ��Һ��
��ʵ�鷽���������ȼ�λͬѧ��������ͼ��ʾ��ʵ�顣
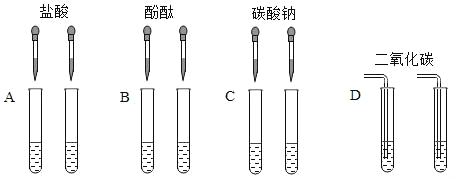
����ش��������⣺
��1�����в��ܴﵽʵ��Ŀ����_____ ������ĸ����
��2��C��ʵ���з�Ӧ�Ļ�ѧ����ʽΪ_____��
��3��������̽����ʵ�����������ͬѧ��A��B��C��D�����Թ��е�����ȫ������ͬһ���ɾ����ձ��У���ַ�Ӧ�õ���ɫ����������Һ���Ը���Һ�ijɷ��ֽ�����̽����
��������⣩����Һ�г�ˮ����̪�������Щ���ʣ�
���������ϣ��Ȼ�����Һ�����ԡ�
����������裩
��NaCl��CaCl2
��NaCl��CaCl2��NaOH
��_____
����˼����չ��
�����������������ֻ��һ��������������_____ ������ţ���������_____��
�ڸ�����ѧ��ѧ֪ʶ����֤�ձ�����Һ�п����е������Ƿ���ڣ�������Щ���ʵ���ʹ�ò�����ɸ�ʵ��_____ ������ĸ����
A ��ɫʯ��ʵ�� B ͭ C ��������Һ D ��������������Һ��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


 NH3��+H2O��+CO2����NH3�ܱ�����Һ���ա�
NH3��+H2O��+CO2����NH3�ܱ�����Һ���ա�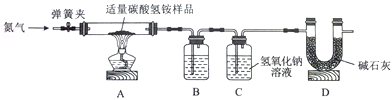
 һ�������������п�Ļ����Һ�У�����������п����������Һ����������������ʱ��ı仯
һ�������������п�Ļ����Һ�У�����������п����������Һ����������������ʱ��ı仯 ʵ�����ø��������ȡ������ʣ��������ʵ����������ʱ��ı仯
ʵ�����ø��������ȡ������ʣ��������ʵ����������ʱ��ı仯 ����������μ�������������Һ����Һ��pH���������������Һ�����ı仯
����������μ�������������Һ����Һ��pH���������������Һ�����ı仯 ���ˮʵ����ԭ����Ŀ�淴Ӧʱ��ı仯
���ˮʵ����ԭ����Ŀ�淴Ӧʱ��ı仯
