��Ŀ����
��2010?��������ģ����Դ�뻷����������ᷢչ�����У�������Ҫ�����ã���1���Ϻ����������ڲ�����Ŀǰ�ڻ������ܷ����һЩ�¼���������Դ���²��ϣ����б�������������������ǣ�����ĸ����
A��̫���ܵ����� B����Դ�ȱý��ܼ�����ʹ��
C�������ῪĻ����ʱ D�����ڽ�ͨ����ʵ�����ŷ�
E���Ż�������ƣ���ǿ������Ȼ�ɹ⣬���������õ�
��2��ú��ʯ�ͺ�����Ҫ�IJ�����������Դ������ʹ�û�ʹ������ŷţ�����ȫ���ů����ѧ�Ҳ��ø��¼�������������̼��������һ����������ϣ�����һ����Ҫ�Ļ���ԭ���ң�C2H4����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��
��3�������������úͿ������ܡ����ܼ�����һ�֣�������Դ������뮵ĺ��ܿ���������Ϊ�ǽ��δ��������Դ���������������Ҫ;��֮һ����֪뮺�����ͬ��Ԫ�أ����ԭ�Ӻ��ڵ�������Ϊ��
���𰸡���������1��ץס�жϵĹؼ���ѡ���е����������ǡ��������������ܡ���
��2������ʯȼ����ú��ʯ�͡���Ȼ�������ݻ�ʯȼ�ϵ�ȼ�ղ��P����ʽ��д������з�����
��3����ʯȼ����Ҫ��ʯ��ú��Ȼ���ȣ��ǵ��������ձ�ʹ�õ���Դ������Դ���Dz�ͬ�ڻ�ʯȼ�ϵ���Դ���ٵ����ܢڳ�ϫ�ܾ���������Դ��Ԫ���Ǿ�����ͬ�˵��������������ͬ��ԭ�ӵ��ܳƣ���Ԫ����ͬ������һ����ͬ��
����⣺��1��ѡ��A��ʹ��̫���ܡ�B�е����ܡ�D�е�̫���ⶼ����������Դ����Լ��Դ��ѡ��Dʵ�����ŷ�����ɫ��ѧ������Ի�������Ⱦ��ѡ��C�뻷������û�й�ϵ������ѡC��
��2������ʯȼ����úʯ����Ȼ����ȼ�ն�����CO2���壬�������ЧӦ������������Ϣ���Ѿ�����Ӧ�������������ˣ�������һ�����������Ը��������غ㶨�ɶԷ�Ӧ��ƽ���ɣ�����ʽΪ��2CO2+6H2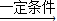 C2H4+4H2O��
C2H4+4H2O��
��3����ʯȼ����Ҫ��ʯ�͡�ú����Ȼ���ȣ��ǵ��������ձ�ʹ�õ���Դ������Դ���Dz�ͬ�ڻ�ʯȼ�ϵ���Դ��̫���ܡ����ܡ����ܡ������ܡ����ܡ�ˮ�ܡ���ϫ�ܾ���������Դ��Ԫ���Ǿ�����ͬ�˵��������������ͬ��ԭ�ӵ��ܳƣ���Ԫ����ͬ������һ����ͬ��������Ϊ1��
�ʴ�Ϊ����1��C����2����Ȼ����������̼��2CO2+6H2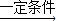 C2H4+4H2O����3�������ܣ����ܡ�ˮ�ܡ���ϫ�ܣ���1��
C2H4+4H2O����3�������ܣ����ܡ�ˮ�ܡ���ϫ�ܣ���1��
�����������Ƕ���Դ�������Ŀ��飬��Ҫ�Ƕ�������Դ��֪ʶ�����˷�������������ʶ�ǿ����⣮
��2������ʯȼ����ú��ʯ�͡���Ȼ�������ݻ�ʯȼ�ϵ�ȼ�ղ��P����ʽ��д������з�����
��3����ʯȼ����Ҫ��ʯ��ú��Ȼ���ȣ��ǵ��������ձ�ʹ�õ���Դ������Դ���Dz�ͬ�ڻ�ʯȼ�ϵ���Դ���ٵ����ܢڳ�ϫ�ܾ���������Դ��Ԫ���Ǿ�����ͬ�˵��������������ͬ��ԭ�ӵ��ܳƣ���Ԫ����ͬ������һ����ͬ��
����⣺��1��ѡ��A��ʹ��̫���ܡ�B�е����ܡ�D�е�̫���ⶼ����������Դ����Լ��Դ��ѡ��Dʵ�����ŷ�����ɫ��ѧ������Ի�������Ⱦ��ѡ��C�뻷������û�й�ϵ������ѡC��
��2������ʯȼ����úʯ����Ȼ����ȼ�ն�����CO2���壬�������ЧӦ������������Ϣ���Ѿ�����Ӧ�������������ˣ�������һ�����������Ը��������غ㶨�ɶԷ�Ӧ��ƽ���ɣ�����ʽΪ��2CO2+6H2
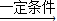 C2H4+4H2O��
C2H4+4H2O����3����ʯȼ����Ҫ��ʯ�͡�ú����Ȼ���ȣ��ǵ��������ձ�ʹ�õ���Դ������Դ���Dz�ͬ�ڻ�ʯȼ�ϵ���Դ��̫���ܡ����ܡ����ܡ������ܡ����ܡ�ˮ�ܡ���ϫ�ܾ���������Դ��Ԫ���Ǿ�����ͬ�˵��������������ͬ��ԭ�ӵ��ܳƣ���Ԫ����ͬ������һ����ͬ��������Ϊ1��
�ʴ�Ϊ����1��C����2����Ȼ����������̼��2CO2+6H2
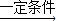 C2H4+4H2O����3�������ܣ����ܡ�ˮ�ܡ���ϫ�ܣ���1��
C2H4+4H2O����3�������ܣ����ܡ�ˮ�ܡ���ϫ�ܣ���1�������������Ƕ���Դ�������Ŀ��飬��Ҫ�Ƕ�������Դ��֪ʶ�����˷�������������ʶ�ǿ����⣮

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ