��Ŀ����
��У��ѧ��ȤС���ͬѧ��̽��������������ʯ��ʯ�����ɳ���ʯ��ʯ��Ʒ��̼��Ƶĺ��������dz�ȡ12.5g����Ʒ�����ձ��У������м�����105.4gϡ���ᣬǡ����ȫ��Ӧ����Ʒ�е����ʲ������ᷴӦ��Ҳ������ˮ��������CO2���ܽ⣩�����ռ���������̼����4.4g����
��1������Ʒ��̼��Ƶ����������Ƕ��٣�
��2��ǡ����ȫ��Ӧ��������Һ���������������Ƕ��٣�
��1������Ʒ��̼��Ƶ����������Ƕ��٣�
��2��ǡ����ȫ��Ӧ��������Һ���������������Ƕ��٣�
��1��80% ��2��10%
��1����12.5gʯ��ʯ��Ʒ�к�CaCO3����Ϊx
��2��CaCO3+2HCl�TCaCl2+CO2��+H2O
��3��100 44
��4��x 4.4g
��5��
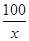 =
=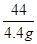 ���õ�x=10g���أ�CaCO3��=
���õ�x=10g���أ�CaCO3��=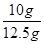 ��100��=80��.
��100��=80��.��2��������Һ���������������ǣ�
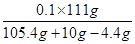 ��100��=10����
��100��=10�������������ݻ�ѧ����ʽ��ؼ��㡣

��ϰ��ϵ�д�
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
�����Ŀ