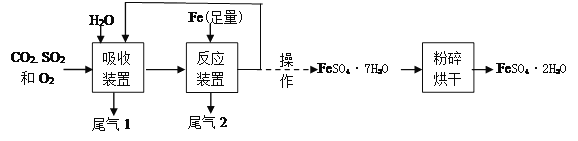
��Ŀ����
����Ŀ�����п�ͼ�е����ʾ�Ϊ���л�ѧ���������ʣ�����A��ʵ������ȡ������̼�Ĺ���ԭ�ϵ���Ҫ�ɷ֣�B�����������ͼ������֮����ת����ϵ����ش�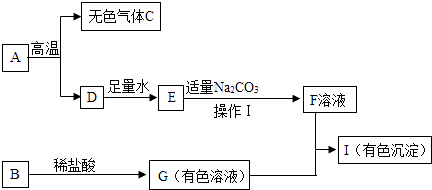
��1��д���������ʵĻ�ѧʽ��A�� �� D�� ��
��2���õ�F��Һ�IJ���I������Ϊ�� ��
��3����I����ɫ��������д��G+F��I�Ļ�ѧ����ʽ�� ��
��4��ָ�����I��Ӧ�Ļ�����������Ӧ��
��5��B��ϡ������ܵ����� �� ���Ӧ��Ӧ����ʽ ��
���𰸡�
��1��CaCO3,CaO
��2������
��3��2NaOH+CuCl2�TCu��OH��2��+2NaCl
��4�����ֽ�
��5����ɫ������ʧ,��Һ����ɫ,CuO+2HCl=CuCl2+H2O
���������⣺��1��A��ʵ������ȡ������̼�Ĺ���ԭ�ϵ���Ҫ�ɷ֣����ݿα�֪ʶ��֪A������̼��ƣ��仯ѧʽΪCaCO3��A̼��Ƹ��·ֽ�������ɫ����C������̼���仯ѧʽΪCO2���Ͱ�ɫ����D�����ƣ���2��D��������ˮ��������E�������ƣ�E����������Һ��̼������Һ��Ӧ����̼��Ƴ�����F����������Һ��̼��Ƴ����ǹ��壬����������Һ��Һ�壬��������ɵĻ�����ʺ���I���˵ķ������룮��3����I����ɫ���������������غ㶨�ɿ�֪��B��G�ж�����ͭԪ�أ����B��ͭ�������G���Ȼ�ͭ��Һ��F����������Һ��G�Ȼ�ͭ��Һ��Ӧ����I��ɫ����������ͭ���Ȼ��ƣ��÷�Ӧ�Ļ�ѧ����ʽΪ��2NaOH+CuCl2�TCu��OH��2��+2NaCl����4������2NaOH+CuCl2�TCu��OH��2��+2NaCl��ͨ�����Ϸ�����֪�����I��Ӧ�Ļ��������Ǹ��ֽⷴӦ����5��B��ϡ���ᷢ����Ӧ�����ܵ������Ǻ�ɫ������ʧ����Һ����ɫ�����Ӧ��Ӧ����ʽ��CuO+2HCl=CuCl2+H2O�����Դ��ǣ���1��CaCO3��CaO�� ��2�����ˣ���3��2NaOH+CuCl2�TCu��OH��2��+2NaCl�� ��4�����ֽ⣻��5����ɫ������ʧ����Һ����ɫ�� CuO+2HCl=CuCl2+H2O��
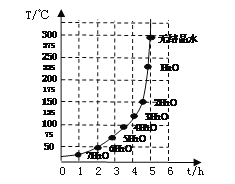
�����㾫����ͨ��������ù��˲�����ע���������д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�����չ��˲���ע�������һ���������͡������������˺���Һ��Ȼ���ǵĿ���ԭ����:�ٳн���Һ���ձ����ɾ����㵹Һ��ʱҺ�������ֽ��Ե����ֽ����ע�⣺a����ƽ b������ c�����ż����Խ����⣮

����Ŀ��ijѧ����A��B��C��D��ֻС��ƿ�зֱ��������ϸ��˿������ʳ��ˮ��ϸ��˿��������ˮ��ϸ��˿��ʳ��ˮ��ϸ��˿����ʹ��˿��ȫ��û��ʳ��ˮ�У�Ȼ��װ�����ͼ��ʾ������װ�ã�ÿ��һ��ʱ�ʲ���������ˮ�������ĸ߶ȣ�������±�1��������������Ϊ������ˮ�������ĸ߶�/cm����ʾ��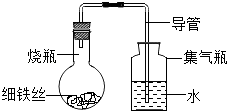
��1 ��ͬʱ��ˮ�������ĸ߶�
ʱ��/Сʱ | 0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
Aƿ��ʢ��ϸ��˿�� | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Bƿ��ʢմ��ʳ��ˮ��ϸ��˿�� | 0 | 0.4 | 1.2 | 3.4 | 5.6 | 7.6 | 9.8 |
Cƿ��ʢմ����ˮ��ϸ��˿�� | 0 | 0 | 0 | 0.3 | 0.8 | 2.0 | 3.5 |
Dƿ��ϸ��˿��ȫ��û��ʳ��ˮ�У� | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
��1��������ˮ��Ϊʲô�������� ��
��2������ʵ���У�������������ɴ�С������˳��Ϊ����С��ƿ�ţ��� ��
��3����������ʵ��Ӱ��������������У� ��
��4��ͨ��������о�������Ϊ���ճ�������Ӧ����α�������������Ʒ�� ��