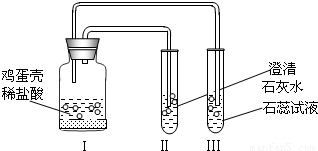
��Ŀ����
��2010?������ģ�⣩���ҹ�����Դ�ṹ�У�ȼúԼռ70%����1��ij��ѧ��ȤС��ԡ�ú̿�Ƿ�����һ������������ۣ��������������ͼ��ʾʵ����вⶨ��Ϊ��ȼú̿�������õ���һ�ֲ��������ǣ�______��
��2�����Dz������Ϻ��ҵ�������һ�λ���������������ʹKMnO4��Һ��ɫ�����Ϻ�ɫ�����ɫ������Ӧ����ʽΪ��5SO2+2KMnO4+2H2O�TK2SO4+2��+2MnSO4����
Ȼ������ʽ��һ�����ʵĻ�ѧʽӡˢ������������������˽��������һ���ᣬ�������ѧ֪ʶ�Ʋ��仯ѧʽ��______����Ӧ�����ɵ�MnSO4��MnԪ�ػ��ϼ�Ϊ______��
��3���������������ӹ��ɵģ��磺KMnO4��K��MnO4���ɣ�K2SO4��K+��SO42-���ɣ��εľ������Һ����ɫͨ�����ɹ����������Ӿ����ģ�
����ݣ�2���в��ĵ��������Ʋ⣺KMnO4��Һ���ֵ��Ϻ�ɫ��������______���ӱ��ֳ����ģ������ӷ��ţ���������______��

���𰸡���������1������ʵ�����е���Ҫ��Դ�����ش�
��2�����������غ㶨�ɻش�ǰһ�գ����ݻ������л��ϼ۵Ĵ�����Ϊ0�ش��һ�գ�
��3�����ݣ�2���е���Ϣ���������������ط�Ӧ�������ᡢ����ء������̣����ߵĻ����Һ����ɫ��˵����������ӡ������ӡ������Ӿ���ɫ��
����⣺��1����ʵ�����У���Ҫ����Դ�Ǿƾ��ƣ���ȼľ̿ʱ���ľ̿���ȣ������þƾ��ƣ�
��2�����������غ㶨�ɣ���ѧ��Ӧǰ��Ԫ�ص��������Ŀ���䣬��Ļ�ѧʽΪ��H2SO4�����ݻ�������Ԫ�ػ��ϼ۵Ĵ�����Ϊ0��MnSO4����Ԫ�صĻ��ϼ�=0-��+6��-��-2��×4=+2��
��3���������������ط�Ӧ�������ᡢ����ء������̣����ݡ�����������ʹKMnO4��Һ��ɫ�����Ϻ�ɫ�����ɫ������Ӧ����ɫ��Һ�д���K+��˵��K+��ɫ�����Ϻ�ɫ����MnO4-���ֳ����ģ�
�ʴ�Ϊ��
��1���ƾ��ƣ�
��2��H2SO4��+2��
��3��MnO4-����Ӧ����ɫ��Һ�д���K+��˵��K+��ɫ�����Ϻ�ɫ����MnO4-���ֳ����ģ�
�������ۺ�ʵ���漰֪ʶ��㣬��ѧ��˼ά����Ҫ��ߣ��ȿ���֪ʶ��ʶ�ǡ����⡢Ǩ�ơ����ã��ֿ���������Աȡ����ɵ�˼ά�������������ʽ�������ѧ��������Ҫ�����п�����������ĿҪ�ص�ѵ����
��2�����������غ㶨�ɻش�ǰһ�գ����ݻ������л��ϼ۵Ĵ�����Ϊ0�ش��һ�գ�
��3�����ݣ�2���е���Ϣ���������������ط�Ӧ�������ᡢ����ء������̣����ߵĻ����Һ����ɫ��˵����������ӡ������ӡ������Ӿ���ɫ��
����⣺��1����ʵ�����У���Ҫ����Դ�Ǿƾ��ƣ���ȼľ̿ʱ���ľ̿���ȣ������þƾ��ƣ�
��2�����������غ㶨�ɣ���ѧ��Ӧǰ��Ԫ�ص��������Ŀ���䣬��Ļ�ѧʽΪ��H2SO4�����ݻ�������Ԫ�ػ��ϼ۵Ĵ�����Ϊ0��MnSO4����Ԫ�صĻ��ϼ�=0-��+6��-��-2��×4=+2��
��3���������������ط�Ӧ�������ᡢ����ء������̣����ݡ�����������ʹKMnO4��Һ��ɫ�����Ϻ�ɫ�����ɫ������Ӧ����ɫ��Һ�д���K+��˵��K+��ɫ�����Ϻ�ɫ����MnO4-���ֳ����ģ�
�ʴ�Ϊ��
��1���ƾ��ƣ�
��2��H2SO4��+2��
��3��MnO4-����Ӧ����ɫ��Һ�д���K+��˵��K+��ɫ�����Ϻ�ɫ����MnO4-���ֳ����ģ�
�������ۺ�ʵ���漰֪ʶ��㣬��ѧ��˼ά����Ҫ��ߣ��ȿ���֪ʶ��ʶ�ǡ����⡢Ǩ�ơ����ã��ֿ���������Աȡ����ɵ�˼ά�������������ʽ�������ѧ��������Ҫ�����п�����������ĿҪ�ص�ѵ����

��ϰ��ϵ�д�
�����Ŀ
��2010?������ģ�⣩��ͼ��ʾ����ƿ��ʢ��x���壬����ѹ�ιܵĽ�ͷa��ʹҺ��y������ƿ�У�����ƿ��������������̨�ϣ��������������z��ˮ�У����ɼ�b���ɼ��ձ���Һ������Ȫһ��������ƿ�У���������ɫ�ĸı䣬��x��y��z�����ǣ� ��

| x���� | y��Һ | z�Լ� | |
| A | O2 | H2SO4 | ��ɫʯ���Լ� |
| B | CO2 | NaOH | ��ɫ��̪��Һ |
| C | CO | Ca��OH��2 | ��ɫ��̪��Һ |
| D | HCl | AgNO3 | ��ɫʯ����Һ |