��Ŀ����
ˮ������֮Դ��������ճ������빤ũҵ�������벻��ˮ��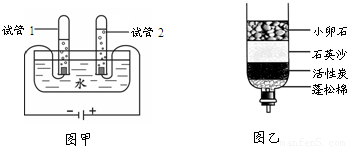
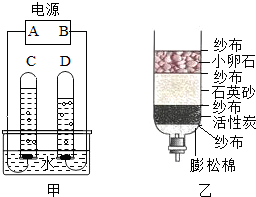
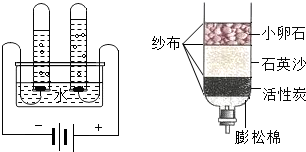
��1����ͼ����ʾ��ͨ��һ��ʱ����Թ�1�����ռ�������Ϊ ����ʵ��˵��ˮ���� ��ɵģ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
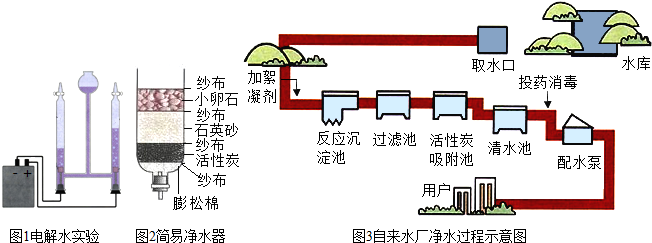
��2��С��������һ������ˮ������ͼ�ң�������ˮ�����л���̿����Ҫ������ �������˾�ˮ���õ���ˮ��Ȼ���Ǵ�ˮ������õ���ˮ�ɲ��õķ����� ��
��3������������Ӳˮ����ˮ�ļ�㷽���� ��
��4������ȼ�ղ�����ˮ������Ϊ��������ȼ�ϣ����Ʊ���ȼ�յĹ����лᷢ�����»�
ѧ��Ӧ����Ӧ���۹��̿�����ͼ��ʾ��

ͼһ��ͼ�������ֵ������У����� �ֺ�����Ԫ�صĻ�����μӷ�Ӧ������������ˮ��������Ϊ ������������������ȱ�ʾ����
���𰸡���������1��������ˮΪ�������ģ�������ˮʵ���ˮ�ľ�������������ʱҪ������ѧ֪ʶ��ʵ�龭�������
��2���ڵ��ˮʵ���У��ɹ۲쵽�������У���������������٣���ʹ�����ǵ�ľ����ȼ����������������࣬��ȼ�գ��������������ٵĶ�����
��3����������Ӳˮ����ˮ�ķ����Ǽ������ˮ������
��4����������������Ƴ������������������������������������������������ܽ�һ���Ƴ�ˮ����Ԫ�غ���Ԫ����ɣ����õľ�ˮ�����г��������ˡ�����������
����⣺��1��ͨ���۲�ͼ�ٿ��Է��֣��Թ�1�����ռ�����������࣬Ӧ������������ô�Թ�2�ռ��ľ�����������������Ԫ����ɣ���������Ԫ����ɣ��ڻ�ѧ�仯��Ԫ������䣬����ˮ����Ԫ�غ���Ԫ����ɣ�
��2������̿����Ҫ�������������ã���ʱ�õ���ˮ����������������ʣ�����õ���ˮ�ɲ��õķ���������
��3��Ӳˮ�������ˮ������ĭ����ˮ�������ˮ�������������ĭ���ʴ𰸣��������ˮ��
��4���۲���ʾ��ͼ֪������Ԫ�صĻ������м����ˮ���֣�����ȼ�յĻ�ѧ����ʽΪCH4+2O2 CO2+2H2O
CO2+2H2O
����������ˮ��������Ϊ16��36����4��9
�ʴ�Ϊ��
��1����������Ԫ�غ���Ԫ�أ�2H2O 2H2��+O2����
2H2��+O2����
��2������������
��3���������ˮ
��4��2��4��9��
�����������ۺϿ���ˮ�ľ������÷������ɻ�ѧʽ�ж����Ԫ�ؼ����û�ѧ�Ľ�����ؼ���Ȼ���֪ʶ�뼼�ܣ�������漰ͼʾ��Ϣ��������Ϣ����Ҫ�����Ϣ�봦����Ϣ��������
��2���ڵ��ˮʵ���У��ɹ۲쵽�������У���������������٣���ʹ�����ǵ�ľ����ȼ����������������࣬��ȼ�գ��������������ٵĶ�����
��3����������Ӳˮ����ˮ�ķ����Ǽ������ˮ������
��4����������������Ƴ������������������������������������������������ܽ�һ���Ƴ�ˮ����Ԫ�غ���Ԫ����ɣ����õľ�ˮ�����г��������ˡ�����������
����⣺��1��ͨ���۲�ͼ�ٿ��Է��֣��Թ�1�����ռ�����������࣬Ӧ������������ô�Թ�2�ռ��ľ�����������������Ԫ����ɣ���������Ԫ����ɣ��ڻ�ѧ�仯��Ԫ������䣬����ˮ����Ԫ�غ���Ԫ����ɣ�
��2������̿����Ҫ�������������ã���ʱ�õ���ˮ����������������ʣ�����õ���ˮ�ɲ��õķ���������
��3��Ӳˮ�������ˮ������ĭ����ˮ�������ˮ�������������ĭ���ʴ𰸣��������ˮ��
��4���۲���ʾ��ͼ֪������Ԫ�صĻ������м����ˮ���֣�����ȼ�յĻ�ѧ����ʽΪCH4+2O2
 CO2+2H2O
CO2+2H2O����������ˮ��������Ϊ16��36����4��9
�ʴ�Ϊ��
��1����������Ԫ�غ���Ԫ�أ�2H2O
 2H2��+O2����
2H2��+O2������2������������
��3���������ˮ
��4��2��4��9��
�����������ۺϿ���ˮ�ľ������÷������ɻ�ѧʽ�ж����Ԫ�ؼ����û�ѧ�Ľ�����ؼ���Ȼ���֪ʶ�뼼�ܣ�������漰ͼʾ��Ϣ��������Ϣ����Ҫ�����Ϣ�봦����Ϣ��������

��ϰ��ϵ�д�
�����Ŀ
 ˮ������֮Դ��������ճ������ũҵ�������벻��ˮ����ͼ����ˮ���ʵ���װ��ͼ���ش��������⣺
ˮ������֮Դ��������ճ������ũҵ�������벻��ˮ����ͼ����ˮ���ʵ���װ��ͼ���ش��������⣺
 ˮ������֮Դ��������ճ������벻��ˮ��
ˮ������֮Դ��������ճ������벻��ˮ��