��Ŀ����
��ͼ��ijƷ�ư״ױ�ǩ�ϵIJ�������˵����ijУ��ȤС����ⶨ��ƿ�״���ȣ�����ĺ������Ƿ����ǩ�ı�ע�����| ԭ�ϣ�ˮ�����ס��ơ���� ��Ʒ����GB18187-2000Һ̬���� �����ڣ�24���� ��;�����衢��� ��ȣ���6.00g/100mL |
��1��NaOH��Һ�ʹ��ᷴӦ����ʽΪ��CH3COOH+NaOH�TCH3COONa+H2O
��2��CH3COOH��NaOH��ȫ�к�ʱ���ټ�һ��NaOH��Һ����Һ�ͳʼ��ԣ����ٵμ�һ��NaOH��ҺԼΪ0.05ml���Բⶨ�����Ӱ���С���ɺ��Բ��ƣ�
��ʵ�鲽�衿
��1����ȡ10ml�ܶȽ���1.0g/ml�İ״ף������ձ��У��ټ���20.0ml����ˮϡ�ͣ������¶ȼƶ�ȡ��ʼ�¶ȣ�
��2����ȡ45.0ml������������Ϊ1.0%�� NaOH��Һ���ܶȽ���1.0g/ml�����õι�ȡNaOH��Һ����εμӵ�ϡ�ͺ�İ״���Һ�У�ͬʱ���Ͻ����ձ��е���Һ�������¶ȼƶ�����______������ǡ����ȫ��Ӧʱʣ��NaOH��Һ5.0ml��
��������˼��
��1��ʵ���У�������ˮϡ�Ͱ״�ʵ����______Ӱ�죨��С����ޡ�����������Ϊ
______��
��2��ʵ��ʱ��Ӧ���ȷ����������������ǡ����ȫ��Ӧ��
______��
�����ݴ���������ͨ�������жϰ״��д��Ậ���Ƿ����ǩ�ı�ע�������Ҫ��д��������̣�
���𰸡���������ʵ�鲽�衿�кͷ�Ӧһ���Ƿ��ȵķ�Ӧ����Ӧʱ�¶Ȼ����ߣ�
�������뷴˼����1����ˮϡ��ʱ���ʵ��������䣬������Ӱ��ʵ��Ľ����
��2�����÷�̪��ָʾ��������Ӧ�Ƿ�ǡ�ý��У�
�����ݴ�������֪�������������Ƶ�������������Ǵ�������������ݻ�ѧ����ʽ�ļ�������
����⣺��ʵ�鲽�衿
��2���кͷ�Ӧһ���Ƿ��ȵķ�Ӧ����Ӧʱ�¶Ȼ����ߣ��ʴ�Ϊ������
��������˼��
��1����Ϊʵ���Ŀ���Dzⶨ�������������ˮϡ��ʱ���ʵ��������䣬������Ӱ��ʵ��Ľ�����ʴ�Ϊ���ޣ�ϡ��ǰ�������������䣮
��2�����÷�̪��ָʾ��������Ӧ�Ƿ�ǡ�ý��У���ʼ��Ӧʱ�������������Ʒ�Ӧ����Һ�����ԣ���̪��ɫ����ǡ�÷�Ӧ�ٵμ�һ������������Һ��ʼ��Է�̪���죬
�ʴ�Ϊ����1.0%��NaOH��Һ�м���1��2�η�̪�������һ��NaOH��Һ����ʹ�ձ��к�ɫ������ȥʱ˵����������������ǡ����ȫ��Ӧ
�����ݴ�����
�μӷ�Ӧ���������Ƶ�����Ϊ����45mL-5mL��×1.0g/L×1.0%�T0.4g
��12g�״��к����������Ϊx
CH3COOH+NaOH�TCH3COONa+H2O
60 40
x 0.4g
 =
= ��x=0.6g
��x=0.6g
��״��д������������Ϊ��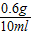 ×100%�T6.0%
×100%�T6.0%
�����6.00g/ml���ϱ�ǩ�ı�ע
������������һ�����Ậ����̽���⣬������Ŀ��������Ϣ������ճ���ѧ���ɽ�����⣬���е����ݴ�������֪һ����Ӧ������һ����Ӧ��ļ��㣬�ǻ�ѧ����ʽ�Ļ����������ͣ�
�������뷴˼����1����ˮϡ��ʱ���ʵ��������䣬������Ӱ��ʵ��Ľ����
��2�����÷�̪��ָʾ��������Ӧ�Ƿ�ǡ�ý��У�
�����ݴ�������֪�������������Ƶ�������������Ǵ�������������ݻ�ѧ����ʽ�ļ�������
����⣺��ʵ�鲽�衿
��2���кͷ�Ӧһ���Ƿ��ȵķ�Ӧ����Ӧʱ�¶Ȼ����ߣ��ʴ�Ϊ������
��������˼��
��1����Ϊʵ���Ŀ���Dzⶨ�������������ˮϡ��ʱ���ʵ��������䣬������Ӱ��ʵ��Ľ�����ʴ�Ϊ���ޣ�ϡ��ǰ�������������䣮
��2�����÷�̪��ָʾ��������Ӧ�Ƿ�ǡ�ý��У���ʼ��Ӧʱ�������������Ʒ�Ӧ����Һ�����ԣ���̪��ɫ����ǡ�÷�Ӧ�ٵμ�һ������������Һ��ʼ��Է�̪���죬
�ʴ�Ϊ����1.0%��NaOH��Һ�м���1��2�η�̪�������һ��NaOH��Һ����ʹ�ձ��к�ɫ������ȥʱ˵����������������ǡ����ȫ��Ӧ
�����ݴ�����
�μӷ�Ӧ���������Ƶ�����Ϊ����45mL-5mL��×1.0g/L×1.0%�T0.4g
��12g�״��к����������Ϊx
CH3COOH+NaOH�TCH3COONa+H2O
60 40
x 0.4g
 =
= ��x=0.6g
��x=0.6g��״��д������������Ϊ��
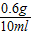 ×100%�T6.0%
×100%�T6.0%�����6.00g/ml���ϱ�ǩ�ı�ע
������������һ�����Ậ����̽���⣬������Ŀ��������Ϣ������ճ���ѧ���ɽ�����⣬���е����ݴ�������֪һ����Ӧ������һ����Ӧ��ļ��㣬�ǻ�ѧ����ʽ�Ļ����������ͣ�

��ϰ��ϵ�д�
 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
�����Ŀ
��ͼ��ijƷ�ư״ױ�ǩ�ϵIJ�������˵����ijУ��ȤС����ⶨ��ƿ�״���ȣ�����ĺ������Ƿ����ǩ�ı�ע�����
| ԭ�ϣ�ˮ�����ס��ơ���� ��Ʒ����GB18187-2000Һ̬���� �����ڣ�24���� ��;�����衢��� ��ȣ���6.00g/100mL��ʵ��ԭ���� ��1��NaOH��Һ�ʹ��ᷴӦ����ʽΪ��CH3COOH+NaOH�TCH3COONa+H2O ��2��CH3COOH��NaOH��ȫ�к�ʱ���ټ�һ��NaOH��Һ����Һ�ͳʼ��ԣ����ٵμ�һ��NaOH��ҺԼΪ0.05ml���Բⶨ�����Ӱ���С���ɺ��Բ��ƣ� ��ʵ�鲽�衿 ��1����ȡ10ml�ܶȽ���1.0g/ml�İ״ף������ձ��У��ټ���20.0ml����ˮϡ�ͣ������¶ȼƶ�ȡ��ʼ�¶ȣ� ��2����ȡ45.0ml������������Ϊ1.0%�� NaOH��Һ���ܶȽ���1.0g/ml�����õι�ȡNaOH��Һ����εμӵ�ϡ�ͺ�İ״���Һ�У�ͬʱ���Ͻ����ձ��е���Һ�������¶ȼƶ����� ��������˼�� ��1��ʵ���У�������ˮϡ�Ͱ״�ʵ���� ��2��ʵ��ʱ��Ӧ���ȷ����������������ǡ����ȫ��Ӧ�� �����ݴ���������ͨ�������жϰ״��д��Ậ���Ƿ����ǩ�ı�ע�������Ҫ��д��������̣� ��ͼ��ijƷ�ư״ױ�ǩ�ϵIJ�������˵����ijУ��ȤС����ⶨ��ƿ�״���ȣ�����ĺ������Ƿ����ǩ�ı�ע�����
��1��NaOH��Һ�ʹ��ᷴӦ����ʽΪ��CH3COOH+NaOH�TCH3COONa+H2O ��2��CH3COOH��NaOH��ȫ�к�ʱ���ټ�һ��NaOH��Һ����Һ�ͳʼ��ԣ����ٵμ�һ��NaOH��ҺԼΪ0.05ml���Բⶨ�����Ӱ���С���ɺ��Բ��ƣ� ��ʵ�鲽�衿 ��1����ȡ10ml�ܶȽ���1.0g/ml�İ״ף������ձ��У��ټ���20.0ml����ˮϡ�ͣ������¶ȼƶ�ȡ��ʼ�¶ȣ� ��2����ȡ45.0ml������������Ϊ1.0%�� NaOH��Һ���ܶȽ���1.0g/ml�����õι�ȡNaOH��Һ����εμӵ�ϡ�ͺ�İ״���Һ�У�ͬʱ���Ͻ����ձ��е���Һ�������¶ȼƶ�����______������ǡ����ȫ��Ӧʱʣ��NaOH��Һ5.0ml�� ��������˼�� ��1��ʵ���У�������ˮϡ�Ͱ״�ʵ����______Ӱ�죨��С����ޡ�����������Ϊ ______�� ��2��ʵ��ʱ��Ӧ���ȷ����������������ǡ����ȫ��Ӧ�� ______�� �����ݴ���������ͨ�������жϰ״��д��Ậ���Ƿ����ǩ�ı�ע�������Ҫ��д��������̣� |