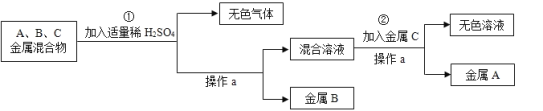
��Ŀ����
����Ŀ�������г���̼��ơ����������������ΪĦ������ijͬѧ��������Ħ����̼��Ƶĺ�������̽����
��ʵ��ԭ�����ⶨCװ�������ɵ�BaCO3������������ͨ������ȷ��������CaCO3������������
���������ϡ�CO2+Ba��OH��2=BaCO3��+H2O�������������ɷ���������ʱ�����������
��ʵ��װ�á�
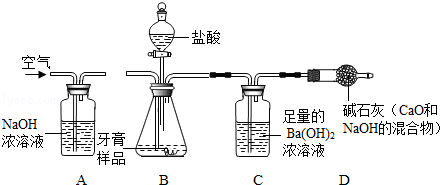
����̽�����̻ش��������⣺
��1��װ��B�з�����Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ����������������ͨ��������������У�������B��C �еķ�Ӧ�ʹ���ַ�Ӧ���� ��
��3����C�й��˳�BaCO3��������IJ����������ձ��� �Ͳ�������
��4��ʵ����ȷ��ȡ����������Ʒ��ÿ��4.0g���������βⶨ���������BaCO3��ƽ������Ϊ1.97g������Ʒ��CaCO3����������Ϊ ��
��5����û��Aװ�ã�ֱ��ͨ�����������CaCO3���������� ���ƫ����ƫС�����䡱����
���𰸡���1��CaCO3+2HCl�TCaCl2+H2O+CO2��
��2��ʹ���ɵĶ�����̼��ȫ������������Һ����
��3��©�� ��4��25% ��5��ƫ��
��������
�����������1��װ��B�У������ܺ�̼��Ʒ�Ӧ��������Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����
���CaCO3+2HCl�TCaCl2+H2O+CO2����
��2����������ͨ�����������ʹ��Ӧ���ɵĶ�����̼��ȫ������������Һ���գ�
���ʹ���ɵĶ�����̼��ȫ������������Һ���գ�
��3����������IJ����������ձ���©���Ͳ�������
���©����
��4����CaCO3+2HCl�TCaCl2+H2O+CO2����CO2+Ba��OH��2=BaCO3��+H2O��֪��CaCO3��BaCO3��
��̼��Ƶ�����ΪX��
CaCO3��BaCO3��
100 197
X 1.97g
![]() =
=![]()
X=1.00g��
̼��Ƶ���������Ϊ��![]() ��100%=25%��
��100%=25%��
���25%��
��5����û��Aװ�ã�ֱ��ͨ�������������еĶ�����̼����Cװ���У��������������գ��Ӷ��������ɵij���ƫ�࣬�������̼���ƫ�࣬��������̼��Ƶ���������ƫ��
���ƫ��