��Ŀ����
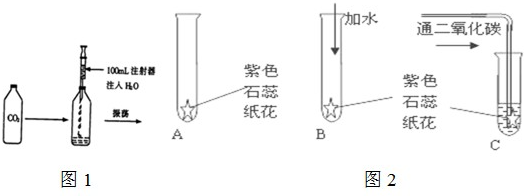
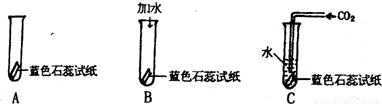
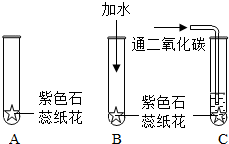 ijͬѧ�����һ����֤̼��������Զ�������̼û�����Ե�ʵ�飮����������ɫʯ����Һ������ֽ��ɹ�ɺ��۳�ֽ����Ȼ����ͼ��ʾ�ֱ���У�
ijͬѧ�����һ����֤̼��������Զ�������̼û�����Ե�ʵ�飮����������ɫʯ����Һ������ֽ��ɹ�ɺ��۳�ֽ����Ȼ����ͼ��ʾ�ֱ���У�
��1����ɫʯ��ֽ������������Һ��______ɫ��
��2����Bʵ���У�������ˮ����ɫʯ��ֽ����______ɫ��Bʵ���������______��
��3����Cʵ���У���ɫʯ��ֽ����______ɫ����һʵ����˵��______��
��4��ͨ������ʵ�飬��δ�ﵽ��֤��Ŀ�ģ���Ҫ�ﵽ��֤��Ŀ�ģ���ĸĽ�������______��
�⣺��1����ɫʯ�������죬����������ʴ𣺺죻
��2��ˮΪ���ԣ�����ʹ���ָʾ��������ɫ�仯��̽�������У�ʵ��B������ʵ��C���бȽϣ��Ƚ�ͨ�������̼�������ɫ����ͬ���ʴ��ϣ����Ա�ʵ�飻
��3��װ��C�ж�����̼ͨ��ˮ�У���ˮ��Ӧ����̼�ᣬʯ�������ɺ�ɫ��
�ʴ𣺺죻C����̼��ʹ��ɫʯ���ɺ�ɫ��
��4����������̽�����̣��ᷢ��̽����û�ж�ʯ��ֻ�Ͷ�����̼����Ӵ�ʱ��������ʵ��̽������˻����ڲ��㣬��������֤�õ�̼��������Զ�����̼�����еĽ��ۣ���Ҫ������һ���̲��У�
�ʴ���Aװ����ͨ������̼��
����������ʯ�������죬ͨ�����Ʊ���̽��������̼���岻��ʹʯ���첻�������ԣ�������ˮ��Ӧ����̼���������ʹʯ���죮̽���ж�����̼��ˮ�DZ�������Ҫ��һ̽����ֻ�ж�����̼��ֻ��ˮ�����ж�����̼����ˮ�����������
������ʯ����Һ�к���ˮ����̽��ֻ�ж�����̼����ʱ�����Ӱ�죬���Դ����̽��������ʹ�õ����ɵ�ʯ��ֽ����
��2��ˮΪ���ԣ�����ʹ���ָʾ��������ɫ�仯��̽�������У�ʵ��B������ʵ��C���бȽϣ��Ƚ�ͨ�������̼�������ɫ����ͬ���ʴ��ϣ����Ա�ʵ�飻
��3��װ��C�ж�����̼ͨ��ˮ�У���ˮ��Ӧ����̼�ᣬʯ�������ɺ�ɫ��
�ʴ𣺺죻C����̼��ʹ��ɫʯ���ɺ�ɫ��
��4����������̽�����̣��ᷢ��̽����û�ж�ʯ��ֻ�Ͷ�����̼����Ӵ�ʱ��������ʵ��̽������˻����ڲ��㣬��������֤�õ�̼��������Զ�����̼�����еĽ��ۣ���Ҫ������һ���̲��У�
�ʴ���Aװ����ͨ������̼��
����������ʯ�������죬ͨ�����Ʊ���̽��������̼���岻��ʹʯ���첻�������ԣ�������ˮ��Ӧ����̼���������ʹʯ���죮̽���ж�����̼��ˮ�DZ�������Ҫ��һ̽����ֻ�ж�����̼��ֻ��ˮ�����ж�����̼����ˮ�����������
������ʯ����Һ�к���ˮ����̽��ֻ�ж�����̼����ʱ�����Ӱ�죬���Դ����̽��������ʹ�õ����ɵ�ʯ��ֽ����

��ϰ��ϵ�д�
�����Ŀ
 21��ijͬѧ�����һ����֤̼��������Զ�������̼û�����Ե�ʵ�飮����������ɫʯ����Һ������ֽ��ɹ�ɺ��۳�ֽ����Ȼ����ͼ��ʾ�ֱ���У�
21��ijͬѧ�����һ����֤̼��������Զ�������̼û�����Ե�ʵ�飮����������ɫʯ����Һ������ֽ��ɹ�ɺ��۳�ֽ����Ȼ����ͼ��ʾ�ֱ���У�