��Ŀ����
ij��ѧʵ��С��Ϊ�ⶨ��ʯ�����ɣ�����x��ֵ����������ʵ�飬�����ᾧˮ������Ʒ��������м��ȣ�����ǰ�ͼ��Ⱥ��г���������ʵ����������ӣ����ȵ�ʱ�䲻���ӳ������ǵ�ʵ�����������£�| ʵ��˳�� �������ӳ�����ʱ�䣩 | ��������/g | |
| ����ǰ | ���Ⱥ� | |
| 1 | 3.44 | 3.26 |
| 2 | 3.44 | 3.12 |
| 3 | 3.44 | 2.90 |
| 4 | 3.44 | 2.90 |
| 5 | 3.44 | 2.80 |
| 6 | 3.44 | 2.78 |
| 7 | 3.44 | 2.72 |
| 8 | 3.44 | 2.72 |
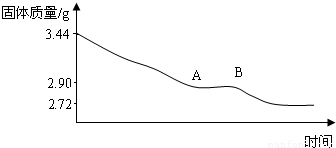
��1������ʵ�����ݣ�ͨ�������ƶ���ʯ��Ļ�ѧʽ��
��2��ͨ���������ͼ���в���AB�ε�ԭ��
���𰸡����������������֪����ǰ�ͼ��Ⱥ������ļ�������������ˮ����������ʵ������֪3.44g CaSO4?xH2O��ȫ�ֽ�õ���ˮCaSO42.72g����֪����ˮ������Ϊ��3.44g-2.72g=0.72g����ˮ������������Ƶ��������������ʯ��Ļ�ѧʽ���ٸ���A-B��ʱʯ�������Ϊ2.90g������CaSO42.72g��H2O0.18g������ͼ���в���AB�ε�ԭ����ʯ�������Ϊ2.90g������CaSO42.72g��H2O0.18g����ʱ�仯ѧʽ�ɱ�ʾΪ2CaSO4?H2O�������ʻ�ѧ�����ȶ������Ȳ��ֽ⣮
�����1���⣺ʯ����ȷֽ���ٵ���������ˮ����������ʵ������֪3.44g CaSO4?xH2O��ȫ�ֽ�õ���ˮCaSO42.72g����
CaSO4?xH2O�TCaSO4+xH2O
136 18x
2.72 0.72
��136��2.72=18x��0.72 ���x=2 ����ʯ��Ļ�ѧʽΪCaSO4?2H2O��
��2����ʵ������֪��A-B��ʱʯ�������Ϊ2.90g������CaSO42.72g��H2O0.18g����ʱ�仯ѧʽ�ɱ�ʾΪ2CaSO4?H2O��
�ʴ�Ϊ��
��1��CaSO4?2H2O��
��2��ԭ����ʯ�������Ϊ2.90g������ͼ�����ݿ�֪�������������䣬˵���Ѿ�û���˽ᾧˮ����CaSO4����Ϊ2.72g������H2O������Ϊ2.90g-2.72g�T0.18g�����������ˮ��������Ϊ��272��18����Ϊ�������ˮ����Է���������Ϊ136��18�����������ǰ��Ӧ�÷���2�����Դ�ʱ�仯ѧʽ�ɱ�ʾΪ2CaSO4?H2O�������ʻ�ѧ�����ȶ������Ȳ��ֽ⣮
��������������Ҫ��ԭ���Ƿ�Ӧǰ�������ļ�������������ˮ���������ٸ���ʵ��������н�һ��������
�����1���⣺ʯ����ȷֽ���ٵ���������ˮ����������ʵ������֪3.44g CaSO4?xH2O��ȫ�ֽ�õ���ˮCaSO42.72g����
CaSO4?xH2O�TCaSO4+xH2O
136 18x
2.72 0.72
��136��2.72=18x��0.72 ���x=2 ����ʯ��Ļ�ѧʽΪCaSO4?2H2O��
��2����ʵ������֪��A-B��ʱʯ�������Ϊ2.90g������CaSO42.72g��H2O0.18g����ʱ�仯ѧʽ�ɱ�ʾΪ2CaSO4?H2O��
�ʴ�Ϊ��
��1��CaSO4?2H2O��
��2��ԭ����ʯ�������Ϊ2.90g������ͼ�����ݿ�֪�������������䣬˵���Ѿ�û���˽ᾧˮ����CaSO4����Ϊ2.72g������H2O������Ϊ2.90g-2.72g�T0.18g�����������ˮ��������Ϊ��272��18����Ϊ�������ˮ����Է���������Ϊ136��18�����������ǰ��Ӧ�÷���2�����Դ�ʱ�仯ѧʽ�ɱ�ʾΪ2CaSO4?H2O�������ʻ�ѧ�����ȶ������Ȳ��ֽ⣮
��������������Ҫ��ԭ���Ƿ�Ӧǰ�������ļ�������������ˮ���������ٸ���ʵ��������н�һ��������

��ϰ��ϵ�д�
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
�����Ŀ
ij��ѧʵ��С��Ϊ�ⶨ��ʯ�����ɣ�����x��ֵ����������ʵ�飬�����ᾧˮ������Ʒ��������м��ȣ�����ǰ�ͼ��Ⱥ��г���������ʵ����������ӣ����ȵ�ʱ�䲻���ӳ������ǵ�ʵ�����������£�
�������ݿɻ��Ƴ�����ͼ��

��1������ʵ�����ݣ�ͨ�������ƶ���ʯ��Ļ�ѧʽ��
��2��ͨ���������ͼ���в���AB�ε�ԭ��
| ʵ��˳�� �������ӳ�����ʱ�䣩 | ��������/g | |
| ����ǰ | ���Ⱥ� | |
| 1 | 3.44 | 3.26 |
| 2 | 3.44 | 3.12 |
| 3 | 3.44 | 2.90 |
| 4 | 3.44 | 2.90 |
| 5 | 3.44 | 2.80 |
| 6 | 3.44 | 2.78 |
| 7 | 3.44 | 2.72 |
| 8 | 3.44 | 2.72 |

��1������ʵ�����ݣ�ͨ�������ƶ���ʯ��Ļ�ѧʽ��
��2��ͨ���������ͼ���в���AB�ε�ԭ��
ij��ѧʵ��С��Ϊ�ⶨ��ʯ�����ɣ�����x��ֵ����������ʵ�飬�����ᾧˮ������Ʒ��������м��ȣ�����ǰ�ͼ��Ⱥ��г���������ʵ����������ӣ����ȵ�ʱ�䲻���ӳ������ǵ�ʵ�����������£�
�������ݿɻ��Ƴ�����ͼ��
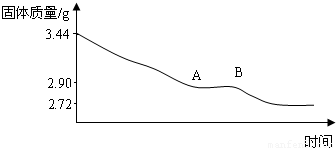
��1������ʵ�����ݣ�ͨ�������ƶ���ʯ��Ļ�ѧʽ��
��2��ͨ���������ͼ���в���AB�ε�ԭ��
| ʵ��˳�� �������ӳ�����ʱ�䣩 | ��������/g | |
| ����ǰ | ���Ⱥ� | |
| 1 | 3.44 | 3.26 |
| 2 | 3.44 | 3.12 |
| 3 | 3.44 | 2.90 |
| 4 | 3.44 | 2.90 |
| 5 | 3.44 | 2.80 |
| 6 | 3.44 | 2.78 |
| 7 | 3.44 | 2.72 |
| 8 | 3.44 | 2.72 |

��1������ʵ�����ݣ�ͨ�������ƶ���ʯ��Ļ�ѧʽ��
��2��ͨ���������ͼ���в���AB�ε�ԭ��
ij��ѧʵ��С��Ϊ�ⶨ��ʯ�����ɣ�����x��ֵ����������ʵ�飬�����ᾧˮ������Ʒ��������м��ȣ�����ǰ�ͼ��Ⱥ��г���������ʵ����������ӣ����ȵ�ʱ�䲻���ӳ������ǵ�ʵ�����������£�
�������ݿɻ��Ƴ�����ͼ��

��1������ʵ�����ݣ�ͨ�������ƶ���ʯ��Ļ�ѧʽ��
��2��ͨ���������ͼ���в���AB�ε�ԭ��
| ʵ��˳�� �������ӳ�����ʱ�䣩 | ��������/g | |
| ����ǰ | ���Ⱥ� | |
| 1 | 3.44 | 3.26 |
| 2 | 3.44 | 3.12 |
| 3 | 3.44 | 2.90 |
| 4 | 3.44 | 2.90 |
| 5 | 3.44 | 2.80 |
| 6 | 3.44 | 2.78 |
| 7 | 3.44 | 2.72 |
| 8 | 3.44 | 2.72 |

��1������ʵ�����ݣ�ͨ�������ƶ���ʯ��Ļ�ѧʽ��
��2��ͨ���������ͼ���в���AB�ε�ԭ��
