��Ŀ����
��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����
��1�����������ϣ���֪��ʽ̼��ͭ�����������ʣ�����Ϊ�������ڻ�ѧ���ʵ���________��
A����ɫ����B��������ˮC�����ȼ�ʽ̼��ͭ������ˮ��������̼������ͭ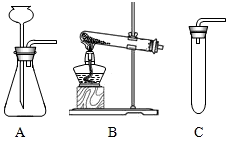
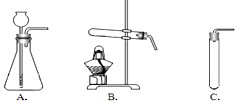
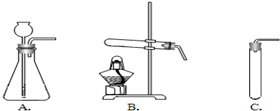
��2�����ȿ�ȸʯ��Ʒʹ֮�ֽ⣬Ӧѡ������________����װ�õ���ţ�װ�ã���������________��
��3���������ɵ��������Ƿ���CO2��Ҫ�õ��Ļ�ѧ�Լ�������________���۲쵽________ʱ��֤��ʵ������CO2������
��4��ѡ�ÿα���ľ̿��ԭ����ͭ��װ�ý���ʵ��ʱ���õ���ɫ���壬һ����Ϊ�ǽ���ͭ��������Ҳ������CҲ�ܽ�CuO��ԭΪ��ɫ����Cu2O��������ͭ����Cu2O+H2SO4=Cu+CuSO4+H2O��������ȤС��ͬѧ������ɽ�һ��ȷ�Ϻ�ɫ����ɷֵ�ʵ�鱨��
| ʵ���顡�١��� | �֡��� | ���� |
| ȡ7.2g��ɫ���壬�����ձ��У������м�������ϡ���ᣬ��ֽ��裬���ã� | ���ޱ仯���� | ֤����ɫ������________�� |
| ����________�� | ֤����ɫ����϶�����________�� | |
| ȡ������Ӧ�����Һ���ˡ�ϴ�ӡ�����ͳ������ù���6.8g�� | -------------- | ȷ�Ϻ�ɫ������________�� |
�⣺��ȸʯ����Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]��
��1��C�ֽⷴӦ���ڻ�ѧ���ʣ�
��2����ʽ̼��ͭ[Cu2��OH��2CO3]Ϊ���壬��Ҫ���Ȳ��ܷ�Ӧ����ѡʵ��װ��B��
��3���ó���ʯ��ˮ�����Ƿ���CO2������ʯ��ˮ����Ǿ�֤��ʵ������CO2������
��4����ϡ�����Ӧ��֤����ɫ������Cu������Һ����ɫ��֤������Cu2O�������˷�Ӧ��Cu2O+H2SO4=Cu+CuSO4+H2O��ȷ�������Cu��Cu2O�Ļ���
�ʴ�Ϊ����1��C
��2��B����Ӧ��Ϊ���壬��Ҫ����
��3������ʯ��ˮ������ʯ��ˮ�����
��4����Cu������Һ����ɫ����Cu2O����Cu��Cu2O�Ļ����
����������̽����ʽ̼��ͭ[Cu2��OH��2CO3]���й����ʣ���Ҫ����ֽ�Ļ�ѧ���ʣ���װ��Ҫѡ����ȵģ��ó���ʯ��ˮ�����Ƿ���CO2����ľ̿��ԭ����ͭ����̽��ʵ�飬Ҫ����ɫ�����Ƿ�����ϡ���ᷴӦ������Ӧ�Ǿ���Cu����������ɫ��Һ���ͺ���Cu2O�ˣ�
����������Ϊ̽���⣬̽����ȸʯ�ijɷ��Լ��й����ʣ�ʵ��̽����ľ̿��ԭ����ͭ������ɣ�
��1��C�ֽⷴӦ���ڻ�ѧ���ʣ�
��2����ʽ̼��ͭ[Cu2��OH��2CO3]Ϊ���壬��Ҫ���Ȳ��ܷ�Ӧ����ѡʵ��װ��B��
��3���ó���ʯ��ˮ�����Ƿ���CO2������ʯ��ˮ����Ǿ�֤��ʵ������CO2������
��4����ϡ�����Ӧ��֤����ɫ������Cu������Һ����ɫ��֤������Cu2O�������˷�Ӧ��Cu2O+H2SO4=Cu+CuSO4+H2O��ȷ�������Cu��Cu2O�Ļ���
�ʴ�Ϊ����1��C
��2��B����Ӧ��Ϊ���壬��Ҫ����
��3������ʯ��ˮ������ʯ��ˮ�����
��4����Cu������Һ����ɫ����Cu2O����Cu��Cu2O�Ļ����
����������̽����ʽ̼��ͭ[Cu2��OH��2CO3]���й����ʣ���Ҫ����ֽ�Ļ�ѧ���ʣ���װ��Ҫѡ����ȵģ��ó���ʯ��ˮ�����Ƿ���CO2����ľ̿��ԭ����ͭ����̽��ʵ�飬Ҫ����ɫ�����Ƿ�����ϡ���ᷴӦ������Ӧ�Ǿ���Cu����������ɫ��Һ���ͺ���Cu2O�ˣ�
����������Ϊ̽���⣬̽����ȸʯ�ijɷ��Լ��й����ʣ�ʵ��̽����ľ̿��ԭ����ͭ������ɣ�

��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ


 ��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����
��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽����� ��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����
��һ�ֿ�ʯ��Ϊ����ȸʯ�������п�ȸ��ë����ɫ���ƣ�������Ҫ�ɷ��Ǽ�ʽ̼��ͭ[Cu2��OH��2CO3]�����ʽ̼��ͭ�к���ͭԪ�أ�ij��ȤС��ͬѧ����δӿ�ȸʯ����������ͭ������Ũ����Ȥ��Ϊ�˲ɼ��������Ŀ�ȸʯ��Ʒ������ʦ��ָ���½���������̽�����