��Ŀ����
17������ǰ���Ƕ��μ���ȫʡ�״�ͳһ����Ļ�ѧʵ��������ܿ��飮��������λͬѧ�μӲ��Ե�����Ͷ�ʵ���˼������ش�������⣮
С��ͬѧ�鵽����Ŀ�ǡ���������ȡ����
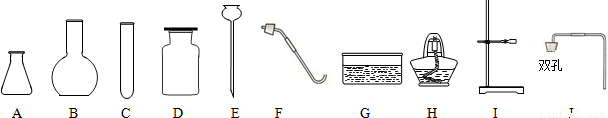
��1������Ҫ���ü��ȸ�����صķ�����ȡ������ʵ��ʱС��ѡ�õ����������ܡ�ë����Ƭ����Ƥ������Ƥ�ܵ�δ������������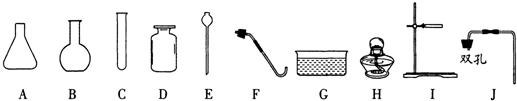
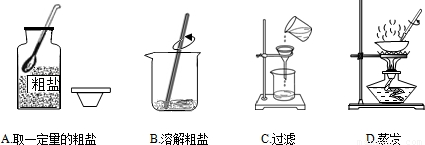
��2��������С��ͬѧ�IJ���ʵ����������в���������
A���ȼ��װ�õ������ԣ����ҩƷ B���ȹ̶��Թܣ�����þƾ���
C���Ƚ��������뼯��ƿ��������Թ� D��ʵ������Ƚ������Ƴ�ˮ�棬��Ϩ��ƾ���
С��ͬѧ�鵽����Ŀ�ǡ����ε��ᴿ����
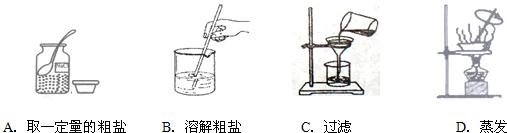
��3��С����ɸ�ʵ��IJ��ֲ���������ͼ��ʾ�����������Դ������
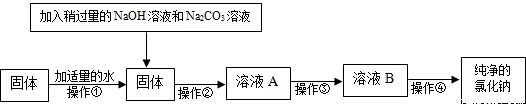
��4��С�ղ���������ϵ�֪�������г�����ɳ�Ȳ����������⣬������������MgCl2��CaCl2�ȿ��������ʣ�������ʵ��õ����������еĹ�������
A�������B�����ʣ�C�������D�������
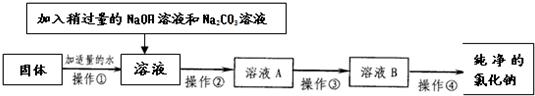
��5��Ϊ�˵õ��������Ȼ��ƣ�С�ս��������еĹ������������´������ٶ�����ֻ��MgCl2��CaCl2���֣���
A���÷����м����NaOH��Һ��Na2CO3��ҺҪ�Թ�����ԭ����
B���������еμӵ��Լ���������
C���������ǽ���ҺB�����������У����Ȳ����Ͻ��裬ֱ��
С��ͬѧ�鵽����Ŀ�ǡ�̽��ij���ε����ʡ���
��6��δ֪��ɫ���������Na2CO3��Na2SO4��NaCl�������е�һ�֣�С����δ֪��ɫ�������Ƴɵ���Һ�еμӷ�̪��Һ���۲쵽��̪����ɫ���μ�ϡ����Ҳ���������μ��Ȼ�����Һ���۲쵽�а�ɫ�������������ɴ˵ó��Ľ����ǣ�δ֪��ɫ���������
��7��ʵ����С���뵽һ�����⣺��ij��ɫ��������Na2CO3��Na2SO4��NaCl�����λ�϶��ɣ���μ������ǵĴ����أ������С�����һ�����ʵ��������ǵĴ��ڣ���������ʵ���У���������ǵ����ȵ���˳����
С��ͬѧ�鵽����Ŀ�ǡ���������ȡ����
��1������Ҫ���ü��ȸ�����صķ�����ȡ������ʵ��ʱС��ѡ�õ����������ܡ�ë����Ƭ����Ƥ������Ƥ�ܵ�δ������������
CHIFGD
��������ĸ��ţ���ͬ��
��2��������С��ͬѧ�IJ���ʵ����������в���������
C
��A���ȼ��װ�õ������ԣ����ҩƷ B���ȹ̶��Թܣ�����þƾ���
C���Ƚ��������뼯��ƿ��������Թ� D��ʵ������Ƚ������Ƴ�ˮ�棬��Ϩ��ƾ���
С��ͬѧ�鵽����Ŀ�ǡ����ε��ᴿ����
��3��С����ɸ�ʵ��IJ��ֲ���������ͼ��ʾ�����������Դ������
A��C
������ĸ��ţ�
��4��С�ղ���������ϵ�֪�������г�����ɳ�Ȳ����������⣬������������MgCl2��CaCl2�ȿ��������ʣ�������ʵ��õ����������еĹ�������
D
������ĸ��ţ�A�������B�����ʣ�C�������D�������
��5��Ϊ�˵õ��������Ȼ��ƣ�С�ս��������еĹ������������´������ٶ�����ֻ��MgCl2��CaCl2���֣���

A���÷����м����NaOH��Һ��Na2CO3��ҺҪ�Թ�����ԭ����
��ֳ���þ���ӡ�������
��B���������еμӵ��Լ���������
ϡ����
��C���������ǽ���ҺB�����������У����Ȳ����Ͻ��裬ֱ��
���ֽ϶����
ʱ��������ֹͣ���ȣ�С��ͬѧ�鵽����Ŀ�ǡ�̽��ij���ε����ʡ���
��6��δ֪��ɫ���������Na2CO3��Na2SO4��NaCl�������е�һ�֣�С����δ֪��ɫ�������Ƴɵ���Һ�еμӷ�̪��Һ���۲쵽��̪����ɫ���μ�ϡ����Ҳ���������μ��Ȼ�����Һ���۲쵽�а�ɫ�������������ɴ˵ó��Ľ����ǣ�δ֪��ɫ���������
Na2SO4��NaCl
����7��ʵ����С���뵽һ�����⣺��ij��ɫ��������Na2CO3��Na2SO4��NaCl�����λ�϶��ɣ���μ������ǵĴ����أ������С�����һ�����ʵ��������ǵĴ��ڣ���������ʵ���У���������ǵ����ȵ���˳����
Na2CO3��Na2SO4��NaCl
����������1������Ҫ���ü��ȸ�����صķ�����ȡ��������ʵ����Ҫ���ȣ��ʿ���ѡ���й�������
��2��������ȡ�����IJ�������ɼ�ס�ھ�����װ��������Ϩ����ע��˳���ܵߵ���
��3���ٳ������Σ�ע��ƿ�ǵķ��ã����ܽ⣺�������Ľ��裬�����ܽ⣻�۹��ˣ�ע��һ������������ԭ���������ò��������Ͻ�����Һ���������ȣ���ֹҺ��ɽ������ȵ��������г��ֽ϶�������ʱ��ֹͣ���ȣ����������������ʹ��Һ���ɣ���ת�ƹ��壻
��4�������⡰�����г�����ɳ�Ȳ����������⣬������������MgCl2��CaCl2�ȿ��������ʡ������֪�����ʷ��ࣻ
��5��A���˽⡰�����NaOH��Һ��Na2CO3��ҺҪ�Թ�����ԭ��
B���������еμӵ��Լ����������������������Һ��̼������Һ��
C�����ա����������̵�ע�����
��6�������⡰С����δ֪��ɫ�������Ƴɵ���Һ�еμӷ�̪��Һ���۲쵽��̪����ɫ���μ�ϡ����Ҳ���������μ��Ȼ�����Һ���۲쵽�а�ɫ���������������Ʋ�����
��7����δ֪��ɫ�������Ƴɵ���Һ�еμӷ�̪��Һ���۲쵽��̪�Ƿ��ɫ���ٷֱ�μ����ᱵ��Һ����������Һ��
��2��������ȡ�����IJ�������ɼ�ס�ھ�����װ��������Ϩ����ע��˳���ܵߵ���
��3���ٳ������Σ�ע��ƿ�ǵķ��ã����ܽ⣺�������Ľ��裬�����ܽ⣻�۹��ˣ�ע��һ������������ԭ���������ò��������Ͻ�����Һ���������ȣ���ֹҺ��ɽ������ȵ��������г��ֽ϶�������ʱ��ֹͣ���ȣ����������������ʹ��Һ���ɣ���ת�ƹ��壻
��4�������⡰�����г�����ɳ�Ȳ����������⣬������������MgCl2��CaCl2�ȿ��������ʡ������֪�����ʷ��ࣻ
��5��A���˽⡰�����NaOH��Һ��Na2CO3��ҺҪ�Թ�����ԭ��
B���������еμӵ��Լ����������������������Һ��̼������Һ��
C�����ա����������̵�ע�����
��6�������⡰С����δ֪��ɫ�������Ƴɵ���Һ�еμӷ�̪��Һ���۲쵽��̪����ɫ���μ�ϡ����Ҳ���������μ��Ȼ�����Һ���۲쵽�а�ɫ���������������Ʋ�����
��7����δ֪��ɫ�������Ƴɵ���Һ�еμӷ�̪��Һ���۲쵽��̪�Ƿ��ɫ���ٷֱ�μ����ᱵ��Һ����������Һ��
����⣺��1������Ҫ���ü��ȸ�����صķ�����ȡ��������ʵ����Ҫ���ȣ�ʵ��ʱС��ѡ�õ����������ܡ�ë����Ƭ����Ƥ������Ƥ�ܵ�δ������������CHIFGD��
��2��������ȡ�����IJ�������ɼ�ס�ھ�����װ��������Ϩ����ע��˳���ܵߵ��������в���������C����Ϊ���ȵ���ա���ֱ�����ݾ���������ð��ʱ����ʼ�ռ���
��3��ע������ᴿ�IJ����ע�������С����ɸ�ʵ��IJ��ֲ���������ͼ��ʾ�����������Դ������A��C��
��4�������⣬С�ղ���������ϵ�֪�������г�����ɳ�Ȳ����������⣬������������MgCl2��CaCl2�ȿ��������ʣ�������ʵ��õ����������еĹ������ڻ���
��5���������֪��A���÷����м����NaOH��Һ��Na2CO3��ҺҪ�Թ�����ԭ���dz�ֳ���þ���ӡ������ӣ�
B���������еμӵ��Լ����������������������Һ��̼������Һ���������еμӵ��Լ������������
C���ȵ��������г��ֽ϶�������ʱ��ֹͣ���ȣ����������������ʹ��Һ���ɣ�
��6��δ֪��ɫ���������Na2CO3��Na2SO4��NaCl�������е�һ�֣�С����δ֪��ɫ�������Ƴɵ���Һ�еμӷ�̪��Һ���۲쵽��̪����ɫ��˵����̼���ƣ��μ�ϡ����Ҳ���������μ��Ȼ�����Һ���۲쵽�а�ɫ����������˵���������ƣ����ɴ˵ó��Ľ����ǣ�δ֪��ɫ���������Na2SO4��NaCl��
��7��ʵ����С���뵽һ�����⣺��ij��ɫ��������Na2CO3��Na2SO4��NaCl�����λ�϶��ɣ���μ������ǵĴ����أ������С�����һ�����ʵ��������ǵĴ��ڣ���δ֪��ɫ�������Ƴɵ���Һ�еμӷ�̪��Һ���۲쵽��̪�Ƿ��ɫ���μ����ᱵ��Һ���۲��Ƿ��а�ɫ�����������μ���������Һ���۲��Ƿ��а�ɫ������������������ʵ���У���������ǵ����ȵ���˳����Na2CO3��Na2SO4��NaCl��
�ʴ�Ϊ����1��CHIFGD����2��C����3��A��C����4��D����5��A��ֳ���þ���ӡ������ӣ�Bϡ���C���ֽ϶���壻��6��Na2SO4��NaCl����7��Na2CO3��Na2SO4��NaCl
��2��������ȡ�����IJ�������ɼ�ס�ھ�����װ��������Ϩ����ע��˳���ܵߵ��������в���������C����Ϊ���ȵ���ա���ֱ�����ݾ���������ð��ʱ����ʼ�ռ���
��3��ע������ᴿ�IJ����ע�������С����ɸ�ʵ��IJ��ֲ���������ͼ��ʾ�����������Դ������A��C��
��4�������⣬С�ղ���������ϵ�֪�������г�����ɳ�Ȳ����������⣬������������MgCl2��CaCl2�ȿ��������ʣ�������ʵ��õ����������еĹ������ڻ���
��5���������֪��A���÷����м����NaOH��Һ��Na2CO3��ҺҪ�Թ�����ԭ���dz�ֳ���þ���ӡ������ӣ�
B���������еμӵ��Լ����������������������Һ��̼������Һ���������еμӵ��Լ������������
C���ȵ��������г��ֽ϶�������ʱ��ֹͣ���ȣ����������������ʹ��Һ���ɣ�
��6��δ֪��ɫ���������Na2CO3��Na2SO4��NaCl�������е�һ�֣�С����δ֪��ɫ�������Ƴɵ���Һ�еμӷ�̪��Һ���۲쵽��̪����ɫ��˵����̼���ƣ��μ�ϡ����Ҳ���������μ��Ȼ�����Һ���۲쵽�а�ɫ����������˵���������ƣ����ɴ˵ó��Ľ����ǣ�δ֪��ɫ���������Na2SO4��NaCl��
��7��ʵ����С���뵽һ�����⣺��ij��ɫ��������Na2CO3��Na2SO4��NaCl�����λ�϶��ɣ���μ������ǵĴ����أ������С�����һ�����ʵ��������ǵĴ��ڣ���δ֪��ɫ�������Ƴɵ���Һ�еμӷ�̪��Һ���۲쵽��̪�Ƿ��ɫ���μ����ᱵ��Һ���۲��Ƿ��а�ɫ�����������μ���������Һ���۲��Ƿ��а�ɫ������������������ʵ���У���������ǵ����ȵ���˳����Na2CO3��Na2SO4��NaCl��
�ʴ�Ϊ����1��CHIFGD����2��C����3��A��C����4��D����5��A��ֳ���þ���ӡ������ӣ�Bϡ���C���ֽ϶���壻��6��Na2SO4��NaCl����7��Na2CO3��Na2SO4��NaCl
�����������Ȼ�������ε��ᴿ�ķ�������ע�����������ȡ������װ�õ�ѡȡ�����ӷ������˽���Ӻ;����ķ�����

��ϰ��ϵ�д�
�����Ŀ