��Ŀ����
��2013?÷�ݣ�2013��3��22���ǵڶ�ʮһ�조����ˮ�ա���ˮ��Դ�ı����ͺ����������ܵ����ǵ��ձ��ע��
��1������ϴ�Ӽ�����Na3P3O10���ˮ��ֲ�������ֳ������ˮ�ʶ���������ᳫʹ������ϴ�Ӽ���Na3P3O10����Ԫ�غ���Ԫ�ص���������
��2����Ȼˮ�ﺬ���������ʣ����������������������˺�����ȷ������о��������о����̶���ߵ���
��3��ClO2����������ˮ��������ȡClO2�Ļ�ѧ����ʽΪ��2NaClO2+X=2ClO2��+2NaCl����X�Ļ�ѧʽΪ
��1������ϴ�Ӽ�����Na3P3O10���ˮ��ֲ�������ֳ������ˮ�ʶ���������ᳫʹ������ϴ�Ӽ���Na3P3O10����Ԫ�غ���Ԫ�ص���������
93��160
93��160
����2����Ȼˮ�ﺬ���������ʣ����������������������˺�����ȷ������о��������о����̶���ߵ���
����
����
����ȥӲˮ�й����Mg2+��Ca2+
Ca2+
�������ӷ��ţ��Ϳɵõ���ˮ����3��ClO2����������ˮ��������ȡClO2�Ļ�ѧ����ʽΪ��2NaClO2+X=2ClO2��+2NaCl����X�Ļ�ѧʽΪ
Cl2
Cl2
��NaClO2����Ԫ�صĻ��ϼ�Ϊ+3
+3
�ۣ���������1�����ݻ������и�Ԫ��������=��ԭ�ӵ����ԭ��������ԭ�Ӹ���֮�ȣ����з������
��2������ķ������Եõ���ˮ�������̶���ߵķ���������
��3�����ݻ�ѧ��Ӧǰ���ԭ�ӵ��������Ŀ������ѧʽ�������ڻ�ѧʽ���������ϼ۵Ĵ�����Ϊ�����ijԪ�صĻ��ϼۣ�
��2������ķ������Եõ���ˮ�������̶���ߵķ���������
��3�����ݻ�ѧ��Ӧǰ���ԭ�ӵ��������Ŀ������ѧʽ�������ڻ�ѧʽ���������ϼ۵Ĵ�����Ϊ�����ijԪ�صĻ��ϼۣ�
����⣺��1��Na3P3O10����Ԫ�غ���Ԫ�ص��������ǣ�31��3������16��10��=93��160��
��2������ķ������Եõ���ˮ�����÷����Ǿ����̶���ߵģ�Ӳˮ�к��й����Mg2+�����ӣ������ӵķ���ΪCa2+��
��3����ѧ��Ӧǰ���ԭ�ӵ��������Ŀ���䣬���ݻ�ѧ����ʽ2NaClO2+X=2ClO2��+2NaCl��֪����������2����ԭ�ӡ�4����ԭ�ӡ�4����ԭ�ӣ���Ӧ������2����ԭ�ӡ�4����ԭ�ӡ�2����ԭ�ӣ�����2����ԭ�ӣ�����X�Ļ�ѧʽΪCl2 ������+1�ۣ�����-2�ۣ���NaClO2����Ԫ�صĻ��ϼ�Ϊx�����ݻ��������������ϼ۵Ĵ�����Ϊ�㣬��+1��+x+��-2����2=0�����x=+3��
�ʴ�Ϊ��
��1��93��160��
��2��Ca2+��
��3��Cl2��+3��
��2������ķ������Եõ���ˮ�����÷����Ǿ����̶���ߵģ�Ӳˮ�к��й����Mg2+�����ӣ������ӵķ���ΪCa2+��
��3����ѧ��Ӧǰ���ԭ�ӵ��������Ŀ���䣬���ݻ�ѧ����ʽ2NaClO2+X=2ClO2��+2NaCl��֪����������2����ԭ�ӡ�4����ԭ�ӡ�4����ԭ�ӣ���Ӧ������2����ԭ�ӡ�4����ԭ�ӡ�2����ԭ�ӣ�����2����ԭ�ӣ�����X�Ļ�ѧʽΪCl2 ������+1�ۣ�����-2�ۣ���NaClO2����Ԫ�صĻ��ϼ�Ϊx�����ݻ��������������ϼ۵Ĵ�����Ϊ�㣬��+1��+x+��-2����2=0�����x=+3��
�ʴ�Ϊ��
��1��93��160��
��2��Ca2+��
��3��Cl2��+3��
�����������ѶȲ�����ͬѧ�ǽ������Ϣ��������û�ѧʽ���йؼ�����з������⡢��������������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
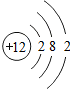 ����þԭ�ӵĺ�����
����þԭ�ӵĺ�����