��Ŀ����
��2004?������þ����Ҫ�Ľ����ṹ���ϣ��������ڷɻ�����ҵ����ˮ��þԪ�ص�����������������Ԫ�غ���Ԫ�أ��ӵ���λ��Ŀǰ�����ϴ�þ���ǴӺ�ˮ����ȡ�ģ�ijУ��ѧ�С���ͬѧ�����ⶨ��ˮ���Ȼ�þ�ĺ������ס��ҡ�����λͬѧ�ֱ����ʵ�飬ʵ�����������±�������ֻ��һλͬѧ�õĺ�ˮ��Ʒ�������������������Һǡ����ȫ��Ӧ���۲�����������ݣ��ش��������⣺| �� | �� | �� | |
| ��ȡ��ˮ��Ʒ��������g�� | 100 | 100 | 150 |
| ����NaOH��Һ��������g�� | 20 | 10 | 10 |
| ��Ӧ�����ij������������g�� | 0.29 | 0.29 | 0.29 |
����ס����ҡ�������
��2�����㺣ˮ��Ʒ��MgCl2����������������������ȷ��0.001��
��3����Ҫ�Ӻ�ˮ����ȡ2.4tþ�������躣ˮ���ٶ֣�
���𰸡���������1����ͼ�����ݿ�֪����ͬѧʵ�����ɳ�����������ȣ�����λͬѧ����ҩƷ���������٣�ʵ��ʱ����Һ����ǡ����ȫ��Ӧ��
��2�������Ȼ�þ���������Ʒ�Ӧ�Ļ�ѧ����ʽ�����ɳ������������г�����ʽ���Ϳɼ����MgCl2��������Ȼ���������������ʽ���㼴�ɣ�
��3�������Ȼ�þ��þ�������������г�����ʽ���Ϳɼ���������躣ˮ��������
����⣺��1����ͼ�����ݿ�֪����ͬѧʵ�����ɳ�����������ȣ�����ͬѧ����ҩƷ���������٣���������Һǡ����ȫ��Ӧ������ͬѧ��ʵ�飻�ʴ�Ϊ���ң�
��2���⣺��MgCl2������Ϊx
MgCl2+2NaOH�TMg��OH��2��+2NaCl
95 58
x 0.29g

��֮�ã�x=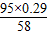 =0.475g��
=0.475g��
��ˮ��MgCl2����������Ϊ��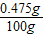 ×100%=0.475%��
×100%=0.475%��
��3���������躣ˮ������Ϊy��
��y×0.475%×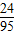 ×100%=2.4t��
×100%=2.4t��
��֮�ã�y=2000t��
�𣺣�2����ˮ��Ʒ��MgCl2����������Ϊ0.475%��
��3�������躣ˮ2000t��
������������Ҫ����ѧ�����û�ѧ����ʽ������������ʽ���м����������
��2�������Ȼ�þ���������Ʒ�Ӧ�Ļ�ѧ����ʽ�����ɳ������������г�����ʽ���Ϳɼ����MgCl2��������Ȼ���������������ʽ���㼴�ɣ�
��3�������Ȼ�þ��þ�������������г�����ʽ���Ϳɼ���������躣ˮ��������
����⣺��1����ͼ�����ݿ�֪����ͬѧʵ�����ɳ�����������ȣ�����ͬѧ����ҩƷ���������٣���������Һǡ����ȫ��Ӧ������ͬѧ��ʵ�飻�ʴ�Ϊ���ң�
��2���⣺��MgCl2������Ϊx
MgCl2+2NaOH�TMg��OH��2��+2NaCl
95 58
x 0.29g

��֮�ã�x=
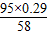 =0.475g��
=0.475g����ˮ��MgCl2����������Ϊ��
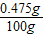 ×100%=0.475%��
×100%=0.475%����3���������躣ˮ������Ϊy��
��y×0.475%×
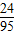 ×100%=2.4t��
×100%=2.4t����֮�ã�y=2000t��
�𣺣�2����ˮ��Ʒ��MgCl2����������Ϊ0.475%��
��3�������躣ˮ2000t��
������������Ҫ����ѧ�����û�ѧ����ʽ������������ʽ���м����������

��ϰ��ϵ�д�
�����Ŀ