��Ŀ����
��2005?�ൺ��ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ���ϱ�����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飻ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У���ַ�Ӧ��ʵ�����ݼ�¼���£�| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �ձ�����ʢ���ʵ�������/g | 181.2 | 204.4 | 228.6 | 253.6 |
��1����һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
��2���ò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%��
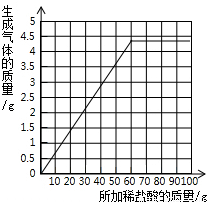
��3������ʵ�����ݣ����±ߵ�����ֽ�ϻ��Ƴ�����ϡ��������������ɵĶ�����̼��������ϵ�����ߣ�����Ҫ��д��������̣�ֻ���������ɣ�

���𰸡���������1�����������غ㶨�ɣ��ڻ�ѧ��Ӧ�У��μӷ�Ӧǰ�����ʵ������ܺ͵��ڷ�Ӧ�����ɸ����ʵ������ܺͣ��ʵ�һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼������=��Ӧǰ�ձ�����ʢ���ʵ������ܺ�-��Ӧ���ձ�����ʢ���ʵ������ܺͣ�
��2����ͼ�����ݿ�֪��ǰ���η�Ӧ��ÿ�������������������1.8g�����Ĵη�Ӧû���������ɣ��ʿ��жϵ����η�Ӧ����ȫ�����������������Ϊ158g+75g-228.6g=4.4g������̼������ϡ���ᷴӦ�Ļ�ѧ����ʽ�����ɵ������������г�����ʽ�����ɼ�������뷴Ӧ��Na2CO3��������Ȼ�����������������=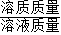 ×100%���㼴�ɣ�
×100%���㼴�ɣ�
����⣺��1����һ�εĽ���У�������̼������Ϊ158.0g+100g÷4-181.2g=1.8g���ʴ�Ϊ��1.8g��
��2��ǰ���η�Ӧ��ÿ�������������������1.8g�����Ĵη�Ӧû���������ɣ��ʿ��жϵ����η�Ӧ����ȫ�����������������Ϊ158g+75g-228.6g=4.4g
����뷴Ӧ��Na2CO3������Ϊx��
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 44
x 4.4g
��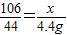 ��
��
��֮�ã�x=10.6g��
��Ʒ��̼���Ƶ���������Ϊ��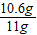 ×100%��96.4%��96%��
×100%��96.4%��96%��
�ʸò�Ʒ��̼���Ƶ����������ϸ�
��3������ǰ�������Լ������̼���ƴ��ڵ�����£�ÿ25g�����Ӧ�Ķ�����̼����Ϊ1.8g��������4.4g������̼ʱ�����ĵ�������Һ������Ϊ61g��֮���ټ��������������ɣ�����ʼʱ������Ϊ�㣬������̼ҲΪ�㣮���Ե�ͼ��
������������Ҫ����ѧ�����û�ѧ����ʽ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������
��2����ͼ�����ݿ�֪��ǰ���η�Ӧ��ÿ�������������������1.8g�����Ĵη�Ӧû���������ɣ��ʿ��жϵ����η�Ӧ����ȫ�����������������Ϊ158g+75g-228.6g=4.4g������̼������ϡ���ᷴӦ�Ļ�ѧ����ʽ�����ɵ������������г�����ʽ�����ɼ�������뷴Ӧ��Na2CO3��������Ȼ�����������������=
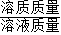 ×100%���㼴�ɣ�
×100%���㼴�ɣ�����⣺��1����һ�εĽ���У�������̼������Ϊ158.0g+100g÷4-181.2g=1.8g���ʴ�Ϊ��1.8g��
��2��ǰ���η�Ӧ��ÿ�������������������1.8g�����Ĵη�Ӧû���������ɣ��ʿ��жϵ����η�Ӧ����ȫ�����������������Ϊ158g+75g-228.6g=4.4g
����뷴Ӧ��Na2CO3������Ϊx��
Na2CO3+2HCl=2NaCl+CO2��+H2O
106 44
x 4.4g
��
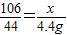 ��
����֮�ã�x=10.6g��
��Ʒ��̼���Ƶ���������Ϊ��
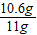 ×100%��96.4%��96%��
×100%��96.4%��96%���ʸò�Ʒ��̼���Ƶ����������ϸ�
��3������ǰ�������Լ������̼���ƴ��ڵ�����£�ÿ25g�����Ӧ�Ķ�����̼����Ϊ1.8g��������4.4g������̼ʱ�����ĵ�������Һ������Ϊ61g��֮���ټ��������������ɣ�����ʼʱ������Ϊ�㣬������̼ҲΪ�㣮���Ե�ͼ��

������������Ҫ����ѧ�����û�ѧ����ʽ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ
��2005?�ൺ��ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ���ϱ�����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飻ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У���ַ�Ӧ��ʵ�����ݼ�¼���£�
����ݴ˷������㣺
��1����һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
��2���ò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%��
��3������ʵ�����ݣ����±ߵ�����ֽ�ϻ��Ƴ�����ϡ��������������ɵĶ�����̼��������ϵ�����ߣ�����Ҫ��д��������̣�ֻ���������ɣ�

| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �ձ�����ʢ���ʵ�������/g | 181.2 | 204.4 | 228.6 | 253.6 |
��1����һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
��2���ò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%��
��3������ʵ�����ݣ����±ߵ�����ֽ�ϻ��Ƴ�����ϡ��������������ɵĶ�����̼��������ϵ�����ߣ�����Ҫ��д��������̣�ֻ���������ɣ�
