��Ŀ����
 1774�꣬��仯ѧ���������о����̿���Ҫ�ɷ���MnO2���Ĺ����У�������Ũ�����ϼ��ȣ�������һ�ֻ���ɫ�����壬��ǿ�ҵĴ̱���ζ��1810�꣬Ӣ����ѧ�Ҵ�άȷ����������Ϊ������Cl2���������о綾���ܶȱȿ�����ԼΪ�����ܶȵ�2.5����������ˮ�������Ļ�ѧ���ʻ��ã�����ˮ����������ȷ�����ѧ��Ӧ������������������Һ��Ӧ�����Ȼ��ƺʹ������ƣ�NaClO����
1774�꣬��仯ѧ���������о����̿���Ҫ�ɷ���MnO2���Ĺ����У�������Ũ�����ϼ��ȣ�������һ�ֻ���ɫ�����壬��ǿ�ҵĴ̱���ζ��1810�꣬Ӣ����ѧ�Ҵ�άȷ����������Ϊ������Cl2���������о綾���ܶȱȿ�����ԼΪ�����ܶȵ�2.5����������ˮ�������Ļ�ѧ���ʻ��ã�����ˮ����������ȷ�����ѧ��Ӧ������������������Һ��Ӧ�����Ȼ��ƺʹ������ƣ�NaClO������1�����շ��������ķ���������ʵ������ȡ��������Ҫ����֮һ���벹�������䷴Ӧ�Ļ�ѧ����ʽ��MnO2+4HCl��Ũ��
| ||
��2�������Ҫ��ø������������ѡ�õĸ������
A����ʯ�� B��Ũ���� C����������
��3������������������Һ��Ӧ�ķ���ʽ
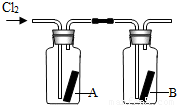
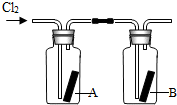
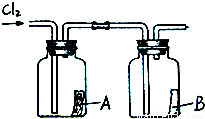
��4����ͼ��ʾ�����������������ͨ��ʢ�и����ֽ���ļ���ƿA��ʢ��ʪ���ֽ���ļ���ƿB������ƿA�еĺ�ֽû�б仯����ƿB�еĺ�ֽ��ɫ�ˣ��������ϣ���ֽ������ϡ������û�б仯���ݴˣ����ܻ��ʲô���ۣ������ƶ�ʹ��ֽ��ɫ�����ʿ�����
������������һ����Ϣ�龰�⣬�����Ҫ����ʵ����Ŀ�ж����������ˣ�ֻҪ�Լ�˼�����ɵó��𰸣���1�������������غ㶨���еķ�Ӧǰ��ԭ�����ࡢ��Ŀ������н��⣻��2��������ж��֣������������������Ũ�����4������Ŀ��֪������ˮ��Ӧ��������ʹ����ᣬ������û��Ư�����ã������Ǵ��������Ư�����ã�
����⣺��1�����������غ㶨�ɿ�֪��Ӧǰ��ԭ�ӵ����ࡢԭ�ӵ���Ŀ���䣬�����Ƴ���������MnCl2��
��2���������峣�õ���Ũ���
��3��������Ѿ���֪�˷�Ӧ�������ֱ����д���ɣ�
��4��������ˮ��Ӧ��������ʹ����ᣬ����û��Ư�����ã����Ǵ��������Ư�����ã�
�ʴ𰸣���1��MnCl2
��2��B
��3��Cl2+2NaOH=NaCl+NaClO+H2O
��4�������� ������ˮ��Ӧ��������ʹ����ᣬ����û��Ư�����ã����Ǵ��������Ư�����ã�
��2���������峣�õ���Ũ���
��3��������Ѿ���֪�˷�Ӧ�������ֱ����д���ɣ�
��4��������ˮ��Ӧ��������ʹ����ᣬ����û��Ư�����ã����Ǵ��������Ư�����ã�
�ʴ𰸣���1��MnCl2
��2��B
��3��Cl2+2NaOH=NaCl+NaClO+H2O
��4�������� ������ˮ��Ӧ��������ʹ����ᣬ����û��Ư�����ã����Ǵ��������Ư�����ã�
��������Ϣ�龰�⿴�ƱȽ������鱾û�е���֪ʶ����ʵ�DZȽ�����������Ŀ����Ҫ������Ŀ����Ч����Ϣ������������

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
 1774�꣬��仯ѧ���������о����̿���Ҫ�ɷ���MnO2���Ĺ����У�������Ũ�����ϼ��ȣ�������һ�ֻ���ɫ�����壬��ǿ�ҵĴ̱���ζ��1810�꣬Ӣ����ѧ�Ҵ�άȷ����������Ϊ������Cl2���������о綾���ܶȱȿ�����ԼΪ�����ܶȵ�2.5����������ˮ�������Ļ�ѧ���ʻ��ã�����ˮ����������ȷ�����ѧ��Ӧ������������������Һ��Ӧ�����Ȼ��ƺʹ������ƣ�NaClO����
1774�꣬��仯ѧ���������о����̿���Ҫ�ɷ���MnO2���Ĺ����У�������Ũ�����ϼ��ȣ�������һ�ֻ���ɫ�����壬��ǿ�ҵĴ̱���ζ��1810�꣬Ӣ����ѧ�Ҵ�άȷ����������Ϊ������Cl2���������о綾���ܶȱȿ�����ԼΪ�����ܶȵ�2.5����������ˮ�������Ļ�ѧ���ʻ��ã�����ˮ����������ȷ�����ѧ��Ӧ������������������Һ��Ӧ�����Ȼ��ƺʹ������ƣ�NaClO����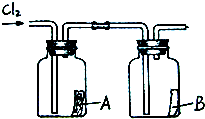
 2H2O+_______+Cl2����
2H2O+_______+Cl2����

 2H2O+______+Cl2����
2H2O+______+Cl2����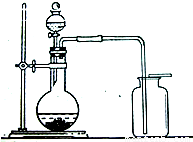
 2H2O+______+Cl2����
2H2O+______+Cl2����