��Ŀ����
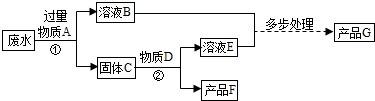
��11�֣�ij��ѧ��ȤС��ͬѧ��ʵ�������ռ���һЩ����ΪFeSO4��CuSO4�ķ�Һ������л��ս���ͭ������������Һ����������·�����

��1����������йط�Ӧ�Ļ�ѧ����ʽ�� ��
��2��������������Լ�Y������Ϊ ��
��3���������X��Ŀ���� ��
��4�������͢��ж��й��˲���������Ҫ�IJ��������� �� �� ��
��5��������о������ˡ�ϴ�ӡ�������Եõ������Ľ���ͭ��ϴ��ʱ�������ͭ�Ƿ���ϴ���ķ����� ��

��1����������йط�Ӧ�Ļ�ѧ����ʽ�� ��
��2��������������Լ�Y������Ϊ ��
��3���������X��Ŀ���� ��
��4�������͢��ж��й��˲���������Ҫ�IJ��������� �� �� ��
��5��������о������ˡ�ϴ�ӡ�������Եõ������Ľ���ͭ��ϴ��ʱ�������ͭ�Ƿ���ϴ���ķ����� ��
��1��Fe+CuSO4===Cu+FeSO4��2�֣���2��ϡ���ᣨ1�֣�
��3��ʹCuSO4��ȫת��ΪFeSO4��2�֣�
��4��©�� ��1�֣� �ձ� ��1�֣� ��������1�֣��������Ⱥ�˳��
��5��ȡ���ϴ��Һ�������μ������Ȼ�����Һ�������ᱵ��Һ������������Һ������������������֤������ͭ��ϴ����3�֣�����д�����1�֣�
��3��ʹCuSO4��ȫת��ΪFeSO4��2�֣�
��4��©�� ��1�֣� �ձ� ��1�֣� ��������1�֣��������Ⱥ�˳��
��5��ȡ���ϴ��Һ�������μ������Ȼ�����Һ�������ᱵ��Һ������������Һ������������������֤������ͭ��ϴ����3�֣�����д�����1�֣�
������Ƶ���ĿҪ��ʵ��Ҫ�ﵽ��Ŀ��������˼��������Ŀ���ǻ��ͭ���ʺ�����������������Ҫ��ͭ����ת��Ϊͭ���ʣ�����Һ����Ҫ�����������������������µ����ʣ����Լ�����ܹ���ͭ����ת��Ϊͭ���ʵ�ֻ���ǵ��������Լ�ʵ����������ʵ������������н��

��ϰ��ϵ�д�
 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
�����Ŀ


 B��������
B�������� C���ֵ���
C���ֵ���
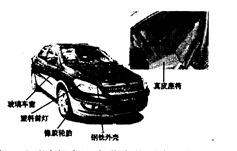
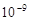 �ף����Ĵ�Сʱ���ͻ���������������벻�������ʣ������صĹ⡢�硢�š��ȡ����ͻ�ѧ�ȷ���ġ��������Ƴ�����ĩ��ͱ���˺�ɫ���Ҳ����磬��еǿ��Ҳ�������ߡ�������Ϣ�жϣ�����˵���������
�ף����Ĵ�Сʱ���ͻ���������������벻�������ʣ������صĹ⡢�硢�š��ȡ����ͻ�ѧ�ȷ���ġ��������Ƴ�����ĩ��ͱ���˺�ɫ���Ҳ����磬��еǿ��Ҳ�������ߡ�������Ϣ�жϣ�����˵���������