��Ŀ����
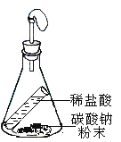
����Ŀ����6�֣��ü���ƿ������ɶ���ʵ�顣���������ʵ��ʾ��ͼ���ش��й����⡣
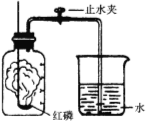
��1��ʵ������ɵ�ʵ���� ��ʵ�����Ĺ��ƿ��װ����Լ��� ��
��2��ʵ����������������װ����ȡ�����Ļ�ѧ����ʽ�� ��
��3��ʵ�����ļ���ƿ��Ԥ�ȼ���������ˮ���������� ��
��4��ʵ�����۲쵽�������� ��
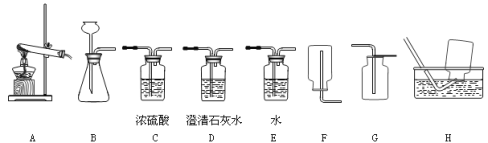
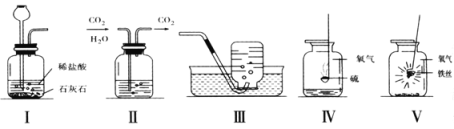
��5����ͼװ�ÿ�����������ռ������顢���Ӻ�����IJ����ȣ�������ɵ�ʵ���� ������ţ���
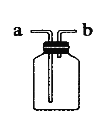
A�������a��ͨ�룬�ռ�����
B��ƿ��װ����ʪ�ĺ�ɫʯ����ֽ�������a��ͨ�룬�����������Ƿ���а���
C��ƿ��װ������������Һ�������b��ͨ�룬����һ����̼�л��еĶ�����̼
D����b�˽���Ͳ��ƿ��װ��ˮ��������������
���𰸡���1��ʵ�����ƶ�����̼��ŨH2SO4 ��2��2H2O2 ===== 2H2O + O2��
��3�������ж���SO2����ֹ��Ⱦ����
��4���������䣬���ɺ�ɫ����
��5��CD
��������
�����������1��ʵ������ʯ��ʯ��ϡ������ȡ������̼��������̼��ˮ����ͨ��Һ����ȥ��ˮ��������ô����Һ��������Ũ���
��2��װ��һ���̹̼����ͣ��ռ�װ������ˮ���ռ���������ѡ�õ����ù���������Һ��ȡ������
��3����ȼ�����ɵ��Ƕ����������������Ⱦ�������ʼ���ƿ�е�ˮ����Ҫ�������ж���SO2����ֹ��Ⱦ������
��4����˿�������о���ȼ�գ��������䣬����һ�ֺڳɹ��壻
��5��������ˮ���ռ����壬��ôˮֻ�ܴ�b���ų��������a��ͨ�룻���dz����ʣ���ôҪ�ѻ������ͨ�뵽��Һ�У�ʹ���dz�ֽӴ���

 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�