��Ŀ����
���������װ�ûش��й����⣺
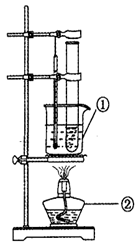
��1��д����ͼ������������ƣ�a______

��2��ʵ���Ҽ��ȸ��������ȡ�����Ļ�ѧ����ʽΪ______��ѡ��ķ���װ��Ϊ______����ͬѧ�������װ��C��ȡ���������������в�������ȷ����______
A����װ��ҩƷ����װ�õ�������
B�����������غ��ڹܿڷ�һС����
C���ȷ��úþƾ��ƣ����ɾƾ��Ƶĸ߶ȹ̶��Թ�
D����ʼ����ǰ�Ƚ����������뼯��ƿ��
E��ֹͣ���Ⱥ��ȴ�ˮ�����ó�����ƿ�����ë��Ƭ��������ʵ��̨��
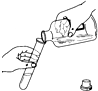
��3��������������ˮ���ܶ���С�����壬ʵ���ҳ���п����ϡ�������Ʊ���������Ӧ����ʽΪ______��ʵ��������ͼFװ�����ռ���������Fװ����װ��ˮ�������______��ͨ�루�c����d������ʵ������Ҫ����980g������������Ϊ10%��ϡ���ᣬ��Ҫ��ȡ������������Ϊ98%��Ũ���ᣨ�ܶ�Ϊ1.84g/cm3 ��______mL������ȡŨ����ʱ�����Ӷ�����������Һ��������������ƫ______�������С����
��4��������ȡ������̼��װ���У�������������ʱ���Ʒ�Ӧ�ķ�����ֹͣ������______��
��1��д����ͼ������������ƣ�a______

��2��ʵ���Ҽ��ȸ��������ȡ�����Ļ�ѧ����ʽΪ______��ѡ��ķ���װ��Ϊ______����ͬѧ�������װ��C��ȡ���������������в�������ȷ����______
A����װ��ҩƷ����װ�õ�������
B�����������غ��ڹܿڷ�һС����
C���ȷ��úþƾ��ƣ����ɾƾ��Ƶĸ߶ȹ̶��Թ�
D����ʼ����ǰ�Ƚ����������뼯��ƿ��
E��ֹͣ���Ⱥ��ȴ�ˮ�����ó�����ƿ�����ë��Ƭ��������ʵ��̨��
��3��������������ˮ���ܶ���С�����壬ʵ���ҳ���п����ϡ�������Ʊ���������Ӧ����ʽΪ______��ʵ��������ͼFװ�����ռ���������Fװ����װ��ˮ�������______��ͨ�루�c����d������ʵ������Ҫ����980g������������Ϊ10%��ϡ���ᣬ��Ҫ��ȡ������������Ϊ98%��Ũ���ᣨ�ܶ�Ϊ1.84g/cm3 ��______mL������ȡŨ����ʱ�����Ӷ�����������Һ��������������ƫ______�������С����
��4��������ȡ������̼��װ���У�������������ʱ���Ʒ�Ӧ�ķ�����ֹͣ������______��

��1������a�ǣ�����©����
��2�����ȸ��������ȡ�����Ļ�ѧ����ʽΪ��2KMnO4
K2MnO4+MnO2+O2������Ϊ���ȹ��������������ѡ��Aװ�ã�
������Ӧ�ȼ��װ�õ������Ժ�װ��ҩƷ����ˮ���ռ�����ʱ����ȵ���������ð�������ð��ʱ�ٿ�ʼ�ռ���ֹͣ���Ⱥ�����ˮ���ò���Ƭ���ϼ���ƿ����ȡ��������
��3����п����ϡ�������Ʊ���������Ӧ����ʽΪZn+H2SO4�TZnSO4+H2��������ͼFװ�����ռ���������Fװ����װ��ˮ�������d���룬
�裬��Ҫ��ȡ������������Ϊ98%��Ũ�������Ϊx��
980g��10%=x��1.84g/cm3��98%
x=54.3mL
��ȡŨ����ʱ�����Ӷ���������Ũ����Ͷ࣬�������������ͱ��
��4��������������ʱ���Ʒ�Ӧ�ķ�����ֹͣ����A����Ϊ�����Һ�岻�ܷ��룮
��Ϊ����1������©��
��2��2KMnO4
K2MnO4+MnO2+O2��ABC
��3��Zn+H2SO4�TZnSO4+H2��d54.3��
��4��A
��2�����ȸ��������ȡ�����Ļ�ѧ����ʽΪ��2KMnO4
| ||
������Ӧ�ȼ��װ�õ������Ժ�װ��ҩƷ����ˮ���ռ�����ʱ����ȵ���������ð�������ð��ʱ�ٿ�ʼ�ռ���ֹͣ���Ⱥ�����ˮ���ò���Ƭ���ϼ���ƿ����ȡ��������
��3����п����ϡ�������Ʊ���������Ӧ����ʽΪZn+H2SO4�TZnSO4+H2��������ͼFװ�����ռ���������Fװ����װ��ˮ�������d���룬
�裬��Ҫ��ȡ������������Ϊ98%��Ũ�������Ϊx��
980g��10%=x��1.84g/cm3��98%
x=54.3mL
��ȡŨ����ʱ�����Ӷ���������Ũ����Ͷ࣬�������������ͱ��
��4��������������ʱ���Ʒ�Ӧ�ķ�����ֹͣ����A����Ϊ�����Һ�岻�ܷ��룮
��Ϊ����1������©��
��2��2KMnO4
| ||
��3��Zn+H2SO4�TZnSO4+H2��d54.3��
��4��A

��ϰ��ϵ�д�
�����Ŀ