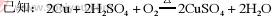��Ŀ����
ͭ�������������õĽ���֮һ��
��1������ͭ��Ʒ�У����ý��������Ե��� ������ĸ��ţ���
A��ͭ�ʽ��� B��ͭ���� C��ͭ���
��2����ʪ����ͭ����ԭ��������ͭ��Һ������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
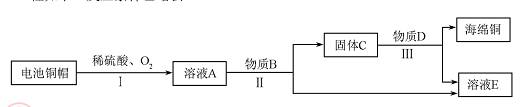
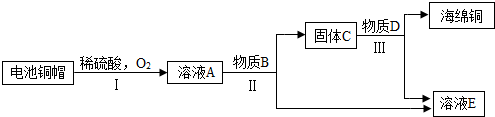
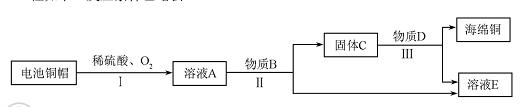
��3�����÷Ͼɵ��ͭñ���� Cu��Zn����ȡ����ͭ��Cu�������õ�����п��Һ����Ҫ�������£���Ӧ��������ȥ����

��֪��2Cu+2H2SO4+O2 2CuSO4+2H2O
2CuSO4+2H2O
�ٹ��̢��з�������������� ��
�ڹ��̢����������������Ӧ�Ļ�ѧ����ʽΪ ��
��A~E �к�ͭ��п����Ԫ�ص������� ������ĸ��ţ���
��1��C ��2��Fe+CuSO4==FeSO4+Cu
��3���ٹ��� ��Zn+H2SO4=ZnSO4+H2�� ��A C
����������1��B���ý����ĵ����� C���ý����ĵ�����
��3����ͨ��������õ��˹������Һ�����Ϊ����
��ͨ����õ���Ŀ����ͬʱ���̢���������������ʻᷢ��Zn+H2SO4=ZnSO4+H2��
�۷Ͼɵ��ͭñ���� Cu��Zn��ͨ�����̣ɵõ�������ҺA����A��һ������ͭ��п����Ԫ�أ���ҺA������Bͨ�����̢�õ�����C����C��һ������ͭ��п����Ԫ��

 ������״Ԫ���Ծ�ϵ�д�
������״Ԫ���Ծ�ϵ�д� ͭ�ʽ��� B��
ͭ�ʽ��� B�� ͭ���� C��
ͭ���� C�� ͭ���
ͭ���

 2CuSO4+2H2O
2CuSO4+2H2O