��Ŀ����
����Ŀ���ס��ҡ�����λͬѧ�������Լ��ֱ����ʵ�飬��ǡ����ȫ��Ӧ�������Լ����������±�����֪��a1+a2+a3=30.6�ˣ�b1+b2+b3=292�ˣ��ֽ��ס��ҡ�����λͬѧ������Һȫ������һ�������ڣ��Ƶô˻����Һ������Ϊ316�ˣ�����
�Լ������� | ��Ӧ��������Һ���� | ||
�� | CaO(��)a1g | 10%����b1g | C1 |
�� | Ca(OH)2(��)a2g | 10%����b2g | C2 |
�� | CaCO3(��)a3g | 10%����b3g | C3 |
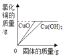
(1)�˻����Һ��������������_______��
(2)a3��ֵ________��
���𰸡�14.1% 15
��������
(1)�������Ȼ�������Ϊx����CaO+2HCl=CaCl2+H2O��Ca(OH)2+2HCl=CaCl2+2H2O��CaCO3+2HCl=CaCl2+H2O+CO2����֪��2HCl��CaCl2��
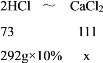
![]() =
=![]() ��
��
x=44.4g��
�˻����Һ��������������Ϊ��![]() ��100%��14.1%��
��100%��14.1%��
�𣺴˻����Һ��������������Ϊ14.1%��
(2)��̼�������Ϊy��
��Ӧ���ɶ�����̼����Ϊ��30.6g+292g-316g=6.6g��
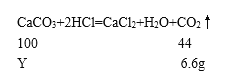
100��y=44��6.6g��
y=15g��
��a3��ֵ��15��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������ͼ������ȷ��ӳ���Ӧ��ϵ����
|
|
|
|
A����ʾ��һ����60 ������ر�����Һ��ȴ������ | B��t �棬��һ�����ı���ʯ��ˮ�м���������ʯ�� | C����ˮͨ��һ��ʱ�� | D����������ȫ��ͬ�������У��ֱ��Cu(OH)2��CuO |
A. AB. BC. CD. D
����Ŀ����ͼA��B�ֱ���þԪ�ء���Ԫ����Ԫ�����ڱ��е���Ϣ��C��D��E���������ӵĽṹʾ��ͼ��������Ԫ�����ڱ���һ���֣�

H | He | ||||||
C | �� | ||||||
Al | �� | �� | |||||
��1��C��D��E����A����ͬ��Ԫ�ص�����_____��
��2��B��Ԫ�����ڱ���_____����ٻ�ڻ�ۣ��Ļ�ѧ�������ƣ�
��3��D���ķ���Ϊ_____��
��4��Ԫ�آڵĺ�����_____�����Ӳ㡣