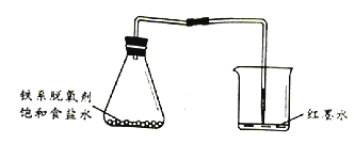
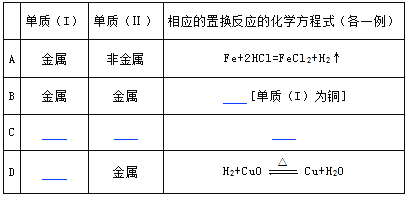
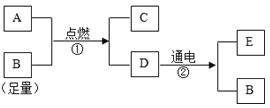
��Ŀ����
����Ŀ��Ϊ�ⶨij����������淋Ĵ��ȣ��������������Ƿ�����ͼ��ǩ����ϣ�С��ȡһ�����õ�����Ʒ���ձ��������Һ�������Ȼ�����Һ��Ӧ����¼�й��������±���


����Ӧ�Ļ�ѧ����ʽΪ��NH4��2SO4+BaCl2 =BaSO4��+2NH4Cl�������ɷ�������ˮ�����μӷ�Ӧ��
��1����Ӧ������BaSO4������Ϊ_____________g��
��2������ȡ������Ʒ������Ϊ15g����ͨ������ȷ���õ���������淋Ĵ����Ƿ����ǩ���������д��������̣�
��3����Ӧǰ����Һ����Ԫ�ص�������_______________����������������������������С������
���𰸡���1��23.3
��2��

��3����С
��������
�����������1�����������غ㶨�ɿ�֪��Ӧǰ����Һ��������ֵΪ���ɵIJ��������ᱵ�����Է�Ӧ������BaSO4������Ϊ45g+55g-76.7g=23.3g��
��2�����������֪����֪��Ϊ���ᱵ��������δ֪��Ϊ����淋���������ͨ����ѧ����ʽ�ж��ߵĹ�ϵ������⡣����������£�

��3��������Ԫ��ת��Ϊ����BaSO4�У������Һ�е���Ԫ��������С��

��ϰ��ϵ�д�
�����Ŀ