��Ŀ����
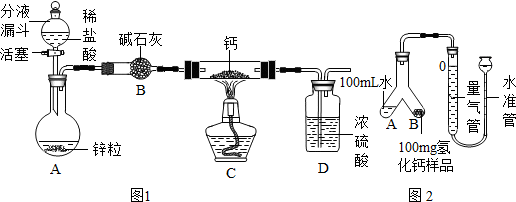
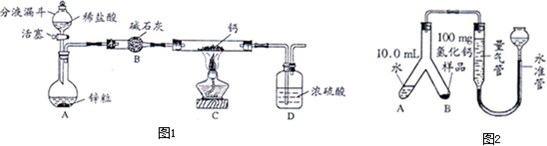
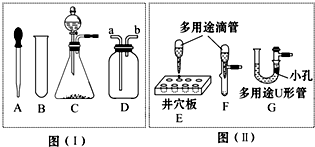 ͼ�����Dz��ֳ��û�ѧ������װ�ã�ͼ�����Dz��֡��͡���ѧ������װ�ã��á��͡�������ʵ�飬���Լ������ͷ����ŷ�����ͨ��������1/10����٣�������ɫ��ѧ��������Ա�ͼ����ͼ���ش��������⣺
ͼ�����Dz��ֳ��û�ѧ������װ�ã�ͼ�����Dz��֡��͡���ѧ������װ�ã��á��͡�������ʵ�飬���Լ������ͷ����ŷ�����ͨ��������1/10����٣�������ɫ��ѧ��������Ա�ͼ����ͼ���ش��������⣺��1��ͼ������A��B�����Ʒֱ���
��ͷ�ι�
��ͷ�ι�
���Թ�
�Թ�
������Dװ����ʢ��ˮ������ˮ���ռ�һƿ����������Ӧ��b
b
�ˣ��a����b����ͨ�룮��2����ͼ�����еġ��͡�������ʵ�飬����ֻ�輫������Һ������Ҳ���٣���ֻ��1��2С�Σ��������ͼ����������ʵ����ŵ���
�٢�
�٢�
������ţ����ٽ�ԼҩƷ���� ������ȫ����ʵ����Ⱦ ���������Ҳ�ܽ�Լȼ��
��3��ͼ�����е�Cװ�õ������൱��ͼ�����е�װ��
F
F
������ĸ����������ʵ������ϵĹ�ͬ�ŵ���ͨ������Һ�������
Һ�������
�����Ʒ�Ӧ�ٶȣ���4��ͼ�����е�Cװ�ú�ͼ�����е�G��������;U�ιܡ�������U�ι�������һ���ײ���С�ľ�֧�Թܣ�������������-Һ����Ӧ��ȡ���壮���ڷ�Ӧ�����зֱ���װ�õ����в��������У����ܳ��ֵ������ǣ�Cװ��
�������
�������
��Gװ��Һ�������룬��Ӧֹͣ
Һ�������룬��Ӧֹͣ
����������1��ֱ��д�������������Լ��������ܶȱ�ˮС���н��
��2���Ӳ����Ƿ��㡢�Ƿ��ԼҩƷ���Ƿ���ܻ������Ƿ�ʹ�õȽǶȷ�����
��3������װ�õ��ص��������жϲ��������
��4�����ݴ���ѹǿ��֪ʶ���з�����
��2���Ӳ����Ƿ��㡢�Ƿ��ԼҩƷ���Ƿ���ܻ������Ƿ�ʹ�õȽǶȷ�����
��3������װ�õ��ص��������жϲ��������
��4�����ݴ���ѹǿ��֪ʶ���з�����
����⣺��1��ͼ��I����AΪ��ͷ�ιܣ�BΪ�Թܣ��������ܶȱ�ˮС���������ˮ���ռ�һƿ����������Ӧ��b��ͨ�룻
��2��ͼ��II��������������Ϊ�����������Խ�ԼҩƷ����Ӱ��ʵ�������ԽϷ�������������ʵ�飬ͬʱ������ȡ�����٣���ʹ���������ȼ��Ҳ�٣����Dz��ܹ���ȫ����ʵ����Ⱦ����ѡ�٢ۣ�
��3������װ�õ��ص����֪����ͼ��I����Cװ��ͨ����Һ©������Һ��ҩƷ������Ҫ����ʱ������������Ҫ����ʱ���رջ������Ӷ��ܹ����Ʒ�Ӧ�ķ�����ֹͣ����ͼ��II���е�Fװ��ͨ����ͷ�ι�����Һ��ҩƷ������Ҫ����ʱ����ѹ��ͷ������Ҫ����ʱ��ֹͣ��ѹ���Ӷ��ܹ����Ʒ�Ӧ�ķ�����ֹͣ����ͼ��I���е�Cװ�ú�ͼ��II���е�Fװ�þ�����ͬ�����ã�
��4��Cװ�ã��������к�װ���ڲ����������Ų���ȥ������ѹǿ�����ܵ����������������;U�ܣ��������к�֧�Թ��ڲ����������Ų���ȥ����֧�Թ��ڵ�����ѹǿ�����Թ��ڵ�Һ��ѹ��ȥ���Ӷ�U�������Һ����������Ӧֹͣ��
�ʴ�Ϊ����1����ͷ�ιܣ��Թܣ�b����2���٢ۣ���3��F��Һ�����������4�����������Һ�������룬��Ӧֹͣ��
��2��ͼ��II��������������Ϊ�����������Խ�ԼҩƷ����Ӱ��ʵ�������ԽϷ�������������ʵ�飬ͬʱ������ȡ�����٣���ʹ���������ȼ��Ҳ�٣����Dz��ܹ���ȫ����ʵ����Ⱦ����ѡ�٢ۣ�
��3������װ�õ��ص����֪����ͼ��I����Cװ��ͨ����Һ©������Һ��ҩƷ������Ҫ����ʱ������������Ҫ����ʱ���رջ������Ӷ��ܹ����Ʒ�Ӧ�ķ�����ֹͣ����ͼ��II���е�Fװ��ͨ����ͷ�ι�����Һ��ҩƷ������Ҫ����ʱ����ѹ��ͷ������Ҫ����ʱ��ֹͣ��ѹ���Ӷ��ܹ����Ʒ�Ӧ�ķ�����ֹͣ����ͼ��I���е�Cװ�ú�ͼ��II���е�Fװ�þ�����ͬ�����ã�
��4��Cװ�ã��������к�װ���ڲ����������Ų���ȥ������ѹǿ�����ܵ����������������;U�ܣ��������к�֧�Թ��ڲ����������Ų���ȥ����֧�Թ��ڵ�����ѹǿ�����Թ��ڵ�Һ��ѹ��ȥ���Ӷ�U�������Һ����������Ӧֹͣ��
�ʴ�Ϊ����1����ͷ�ιܣ��Թܣ�b����2���٢ۣ���3��F��Һ�����������4�����������Һ�������룬��Ӧֹͣ��
��������������˷ḻ��ʵ���������֪ʶ��Ҫ��ѧ����Ϥʵ��������ͬʱ��ʵ��ԭ���н���̵���ʶ�����⣮

��ϰ��ϵ�д�
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
�����Ŀ