��Ŀ����
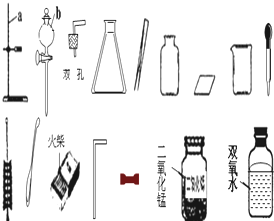
 ����ѧʵ��ʱ��ʵ��̨�ϵ�ҩƷӦ��������ذڷţ���һ�λ�ѧ��ȤС���У�ʵ��̨�ϰڷ�������ҩƷ�������ᣬ�����ᣬ���������أ����������ƣ�������������
����ѧʵ��ʱ��ʵ��̨�ϵ�ҩƷӦ��������ذڷţ���һ�λ�ѧ��ȤС���У�ʵ��̨�ϰڷ�������ҩƷ�������ᣬ�����ᣬ���������أ����������ƣ�������������̼���ƻ��Ȼ��ƻ�������
̼���ƻ��Ȼ��ƻ�������
��þ�� ��ͭ�����к��߿հ״����Լ�ƿ�ı�ǩ������ͼ��ʾ����1�����Ƚ�ͭ�����Ľ������ǿ������ѡ������ҩƷ�е�
��
��
����ʵ�飨ѡ����ţ�����2��������ЩҩƷ�����ڷ��ú����ױ��ʣ��Ծ�һ����˵�����ʵ�ԭ��
Ca��OH��2+CO2=CaCO3��+H2O
Ca��OH��2+CO2=CaCO3��+H2O
���û�ѧ����ʽ��ʾ������3��ͬѧ����̽����ǩ�����ҩƷ�ijɷ֣�
[����˼��]����ҩƷ����ڷŵ�ԭ��ҩƷ������
C
C
��A���� B���� C���� D������
[��������]A��������Na2CO3��Һ
B����������NaCl��Һ
[��Ʋ�ʵ��]
��С����ⶨ����Һ�����ȣ�����ʹ�����������е�
C
C
A��ʯ����Һ B��PH��ֽ C����̪��Һ
��С����ø���Һ��PHֵ����7��Сǿѡ�����ڷŵ��Լ�����С����ʵ�������ͨ����һ����ʵ��ȷ������Na2CO3��Һ�����㲹ȫСǿ��ʵ�鱨�森
| ѡ���Լ���������ţ� | ʵ������ | ���� |
�� �� |
�������� ���� | ԭ�Լ���Na2CO3��Һ |
NaHCO3
NaHCO3
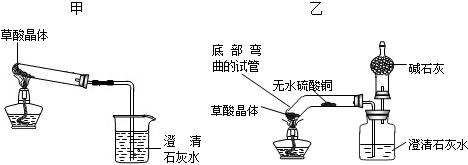
��Һ������������ͭ�Ļ�Ա���ǿ��ѡȡ��ͼ�е�ҩƷ�����ʵ�����ͭ�����Ļ�Թ�ϵ������ͼ�����ʵ����ʣ��ж�ҩƷ���ڷ���ʱ����������й����巢����Ӧ�����ʵ�ҩƷ��˵�����ʵ�ԭ����˼�������ݱ�ǩ�пɼ����ֵ���ʾ��Ϣ�����öԸ������ʵ�����ص����ʶ�������ҩƷ��������𣻸���̼���Ƶ����ʼ��仯���ɣ�ѡȡͼʾ��ҩƷ�����ʵ�����ҩƷΪ̼���ƣ���˼�����ۣ����ݶ��������ᷴӦ�ų�������̼�����ʵ��˽⣬˵����̼�������������ᷴӦ�ų�������̼����һ�����Σ�
����⣺����ҩƷ����ڷŵ�ԭ�����ᡢ��Ρ��������ʷ��࣬��ҩƷ�������Σ��ʴ�Ϊ��̼���ƻ��Ȼ��ƻ�������
��1������ͭ�Ļ�Ա���ǿ��ѡȡ��ͼ�е�ҩƷ�����ʵ�����ͭ�����Ļ�Թ�ϵ�����ѡ����������Һ���ʴ�Ϊ����
��2������ҩƷ����ڷŵ�ԭ��ҩƷ���ܣ��ʴ�Ϊ��Ca��OH��2+CO2=CaCO3��+H2O
��3������ҩƷ����ڷŵ�ԭ�����ᡢ��Ρ��������ʷ��࣬��ҩƷ�������ࣻС����ⶨ����Һ�����ȣ�����ʹ�����������еķ�̪����Ϊ��̪�������Ի�������Һ������ɫ������̼���ƿ������ᷴӦ�ų���ʹ����ʯ��ˮ����ǵĶ�����̼���壬��ˣ���ѡȡ��ͼ�Լ��е�ϡ�����ϡ���ᣬ������Ƽ���ʵ�飻[��˼������]С�մ�̼�����Ƶ���ҺҲ�ʼ��ԣ�������Ҳ���Էų�������̼���壬��ˣ�Сΰͬѧ��Ϊ����ҺҲ����Ϊ̼��������Һ���ʴ�Ϊ��C��C���٣�NaHCO3��
��1������ͭ�Ļ�Ա���ǿ��ѡȡ��ͼ�е�ҩƷ�����ʵ�����ͭ�����Ļ�Թ�ϵ�����ѡ����������Һ���ʴ�Ϊ����
��2������ҩƷ����ڷŵ�ԭ��ҩƷ���ܣ��ʴ�Ϊ��Ca��OH��2+CO2=CaCO3��+H2O
��3������ҩƷ����ڷŵ�ԭ�����ᡢ��Ρ��������ʷ��࣬��ҩƷ�������ࣻС����ⶨ����Һ�����ȣ�����ʹ�����������еķ�̪����Ϊ��̪�������Ի�������Һ������ɫ������̼���ƿ������ᷴӦ�ų���ʹ����ʯ��ˮ����ǵĶ�����̼���壬��ˣ���ѡȡ��ͼ�Լ��е�ϡ�����ϡ���ᣬ������Ƽ���ʵ�飻[��˼������]С�մ�̼�����Ƶ���ҺҲ�ʼ��ԣ�������Ҳ���Էų�������̼���壬��ˣ�Сΰͬѧ��Ϊ����ҺҲ����Ϊ̼��������Һ���ʴ�Ϊ��C��C���٣�NaHCO3��
������������к�ǿ���ۺ��ԣ��ڽ������ʱ���ֳ����û�ѧ֪ʶ���з����������������������Ҳ���ֳ�ѧ����ʵ�鼼�ܣ��й�ʵ�鷽������ƺͶ�ʵ�鷽�����������п����ȵ�֮һ�����ʵ�鷽��ʱ��Ҫע�������ٵ�ҩƷ����ķ�������������Ҫ������ʵ�����У�

��ϰ��ϵ�д�
 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
�����Ŀ