МвДҝДЪИЭ
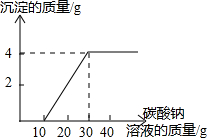 КөСйКТЦЖИЎ¶юСх»ҜМјә󣬶ԻШКХөДСОЛбәНВИ»ҜёЖ»мәПИЬТәЈЁІ»ҝјВЗЖдЛыФУЦКЈ©ҪшРРБЛТФПВКөСйЈәИЎ100gёГИЬТәУЪЙХұӯЦРЈ¬өОИл30gИЬЦКЦКБҝ·ЦКэОӘ21.2%өДМјЛбДЖИЬТәЈ¬ід·Ц·ҙУҰәу№эВЛЈ®өОИлМјЛбДЖИЬТәЦКБҝУлЙъіЙОпіБөнЦКБҝөД№ШПөИзНјЛщКҫЈ®ЗуЈә
КөСйКТЦЖИЎ¶юСх»ҜМјә󣬶ԻШКХөДСОЛбәНВИ»ҜёЖ»мәПИЬТәЈЁІ»ҝјВЗЖдЛыФУЦКЈ©ҪшРРБЛТФПВКөСйЈәИЎ100gёГИЬТәУЪЙХұӯЦРЈ¬өОИл30gИЬЦКЦКБҝ·ЦКэОӘ21.2%өДМјЛбДЖИЬТәЈ¬ід·Ц·ҙУҰәу№эВЛЈ®өОИлМјЛбДЖИЬТәЦКБҝУлЙъіЙОпіБөнЦКБҝөД№ШПөИзНјЛщКҫЈ®ЗуЈәЈЁ1Ј©·ҙУҰНкИ«әуЙъіЙМјЛбёЖ
4
4
ҝЛЈ®ЈЁ2Ј©ЗуЛщИЎ100g»мәПИЬТәЦРHClөДЦКБҝ·ЦКэКЗ¶аЙЩЈҝ
ЈЁ3Ј©КөСйҪбКшәуЈ¬Ҫ«№эВЛЛщөГөДИЬТәХфёЙЈ¬өГөҪ№ММеөДЦКБҝКЗ¶аЙЩҝЛЈҝ
·ЦОцЈәЈЁ1Ј©УЙНјКҫҝЙЦӘ·ҙУҰНкИ«әуЙъіЙіБөнөДЦКБҝҫНКЗМјЛбёЖөДЦКБҝҪшРРҪвҙрЈ»
ЈЁ2Ј©УЙНјКҫҝЙЦӘҝӘКјөОИл10gМјЛбДЖИЬТәКұГ»УРіБөнЙъіЙЈ¬ЛөГчКЗУлСОЛб·ўЙъ·ҙУҰЈ®№КУлСОЛб·ўЙъ·ҙУҰөДМјЛбДЖөДЦКБҝОӘ10gЎБ21.2%=2.12gЈ¬И»әуёщҫЭ»ҜС§·ҪіМКҪЈ¬өГіцёчОпЦКЦ®јдөДЦКБҝұИЈ¬БРіцұИАэКҪЈ¬ҫНҝЙјЖЛгіц»мәПИЬТәЦРВИ»ҜЗвөДЦКБҝәНЙъіЙВИ»ҜДЖөДЦКБҝөДЦКБҝЈ®И»әуёщҫЭИЬЦКЦКБҝ·ЦКэ№«КҪҫНҝЙјЖЛгіц»мәПИЬТәЦРВИ»ҜЗвөДЦКБҝ·ЦКэЈ»
ЈЁ3Ј©УЙМвТвҝЙЦӘЈ¬Ҫ«№эВЛЛщөГөДИЬТәХфёЙЈ¬өГөҪ№ММеөДЦКБҝјҙЙъіЙөДNaClөДЦКБҝЈ®ёщҫЭНјКҫҝЙЦӘЈ¬УлВИ»ҜёЖ·ҙУҰөДМјЛбДЖИЬТәөДЦКБҝОӘ20gЈ¬ёщҫЭИЬТәөДИЬЦКЦКБҝ·ЦКэјЖЛгіцМјЛбДЖөДЦКБҝЈ®И»әуёщҫЭ»ҜС§·ҪіМКҪЈ¬өГіцёчОпЦКЦ®јдөДЦКБҝұИЈ¬БРіцұИАэКҪЈ¬ҫНҝЙјЖЛгіцЙъіЙВИ»ҜДЖөДЦКБҝЈ®
ЈЁ2Ј©УЙНјКҫҝЙЦӘҝӘКјөОИл10gМјЛбДЖИЬТәКұГ»УРіБөнЙъіЙЈ¬ЛөГчКЗУлСОЛб·ўЙъ·ҙУҰЈ®№КУлСОЛб·ўЙъ·ҙУҰөДМјЛбДЖөДЦКБҝОӘ10gЎБ21.2%=2.12gЈ¬И»әуёщҫЭ»ҜС§·ҪіМКҪЈ¬өГіцёчОпЦКЦ®јдөДЦКБҝұИЈ¬БРіцұИАэКҪЈ¬ҫНҝЙјЖЛгіц»мәПИЬТәЦРВИ»ҜЗвөДЦКБҝәНЙъіЙВИ»ҜДЖөДЦКБҝөДЦКБҝЈ®И»әуёщҫЭИЬЦКЦКБҝ·ЦКэ№«КҪҫНҝЙјЖЛгіц»мәПИЬТәЦРВИ»ҜЗвөДЦКБҝ·ЦКэЈ»
ЈЁ3Ј©УЙМвТвҝЙЦӘЈ¬Ҫ«№эВЛЛщөГөДИЬТәХфёЙЈ¬өГөҪ№ММеөДЦКБҝјҙЙъіЙөДNaClөДЦКБҝЈ®ёщҫЭНјКҫҝЙЦӘЈ¬УлВИ»ҜёЖ·ҙУҰөДМјЛбДЖИЬТәөДЦКБҝОӘ20gЈ¬ёщҫЭИЬТәөДИЬЦКЦКБҝ·ЦКэјЖЛгіцМјЛбДЖөДЦКБҝЈ®И»әуёщҫЭ»ҜС§·ҪіМКҪЈ¬өГіцёчОпЦКЦ®јдөДЦКБҝұИЈ¬БРіцұИАэКҪЈ¬ҫНҝЙјЖЛгіцЙъіЙВИ»ҜДЖөДЦКБҝЈ®
ҪвҙрЈәҪвЈәЈЁ1Ј©УЙНјКҫҝЙЦӘ·ҙУҰНкИ«әуЙъіЙіБөнөДЦКБҝҫНКЗМјЛбёЖөДЦКБҝЈ¬ОӘ4gЈ»№КМоЈә4Ј»
ЈЁ2Ј©ёщҫЭНјКҫҝЙЦӘЈ¬УлСОЛб·ҙУҰөДМјЛбДЖөДЦКБҝОӘЈә10gЎБ21.2%=2.12gЈ®
Йи100g»мәПИЬТәЦРВИ»ҜЗвөДЦКБҝОӘxЈ¬ЙъіЙВИ»ҜДЖөДЦКБҝОӘyЈ¬
Na2CO3+2HCl=2NaCl+H2O+CO2Ўь
106 73 117
2.12g x y
=
x=1.46g
=
y=2.34gЈ¬
»мәПИЬТәЦРВИ»ҜЗвөДЦКБҝ·ЦКэОӘЈә
ЎБ100%=1.46%Ј®
ЈЁ3Ј©ёщҫЭНјКҫУлВИ»ҜёЖ·ҙУҰөДМјЛбДЖөДЦКБҝОӘЈә20gЎБ21.2%=4.24gЈ¬
ЙиЙъіЙВИ»ҜДЖөДЦКБҝОӘzЈ®
Na2CO3+CaCl2=2NaCl+CaCO3Ўэ
106 117
4.24g z
=
z=4.68g
КөСйҪбКшәуЈ¬Ҫ«№эВЛЛщөГөДИЬТәХфёЙЈ¬өГөҪ№ММеөДЦКБҝОӘЈә2.34g+4.68g+2.12g=7.02gЈ®
ҙрЈәЈЁ2Ј©ЛщИЎ100g»мәПИЬТәЦРHClөДЦКБҝ·ЦКэКЗ1.46%Ј»ЈЁ3Ј©КөСйҪбКшәуЈ¬Ҫ«№эВЛЛщөГөДИЬТәХфёЙЈ¬өГөҪ№ММеөДЦКБҝОӘ7.02gЈ®
ЈЁ2Ј©ёщҫЭНјКҫҝЙЦӘЈ¬УлСОЛб·ҙУҰөДМјЛбДЖөДЦКБҝОӘЈә10gЎБ21.2%=2.12gЈ®
Йи100g»мәПИЬТәЦРВИ»ҜЗвөДЦКБҝОӘxЈ¬ЙъіЙВИ»ҜДЖөДЦКБҝОӘyЈ¬
Na2CO3+2HCl=2NaCl+H2O+CO2Ўь
106 73 117
2.12g x y
| 106 |
| 2.12g |
| 73 |
| x |
x=1.46g
| 106 |
| 2.12g |
| 117 |
| y |
y=2.34gЈ¬
»мәПИЬТәЦРВИ»ҜЗвөДЦКБҝ·ЦКэОӘЈә
| 1.46g |
| 100g |
ЈЁ3Ј©ёщҫЭНјКҫУлВИ»ҜёЖ·ҙУҰөДМјЛбДЖөДЦКБҝОӘЈә20gЎБ21.2%=4.24gЈ¬
ЙиЙъіЙВИ»ҜДЖөДЦКБҝОӘzЈ®
Na2CO3+CaCl2=2NaCl+CaCO3Ўэ
106 117
4.24g z
| 106 |
| 4.24g |
| 117 |
| z |
z=4.68g
КөСйҪбКшәуЈ¬Ҫ«№эВЛЛщөГөДИЬТәХфёЙЈ¬өГөҪ№ММеөДЦКБҝОӘЈә2.34g+4.68g+2.12g=7.02gЈ®
ҙрЈәЈЁ2Ј©ЛщИЎ100g»мәПИЬТәЦРHClөДЦКБҝ·ЦКэКЗ1.46%Ј»ЈЁ3Ј©КөСйҪбКшәуЈ¬Ҫ«№эВЛЛщөГөДИЬТәХфёЙЈ¬өГөҪ№ММеөДЦКБҝОӘ7.02gЈ®
өгЖАЈәұҫМвЦчТӘҝјІйС§ЙъФЛУГ»ҜС§·ҪіМКҪәНИЬЦКЦКБҝ·ЦКэ№«КҪҪшРРјЖЛгөДДЬБҰЈ®

Б·П°ІбПөБРҙр°ё
 ФД¶БҝміөПөБРҙр°ё
ФД¶БҝміөПөБРҙр°ё
Па№ШМвДҝ