��Ŀ����
��2012?�Ӷ���һģ��A��B��C��D��E��F��G������������ͼ��ʾ���ת����ϵ��
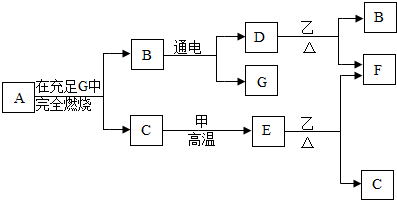
��֪�������£����ʼס��������Ҿ�Ϊ��ɫ���壬��F��Ϊ��ɫ���嵥�ʣ�A��C��D��E��G��Ϊ��ɫ���壬������A��ֻ��������Ԫ�أ����ǵ�������Ϊ3��1����ش��������⣺
��1��д��A��E�����������ʵĻ�ѧʽ��
A
��2��д��A�ڳ���G����ȫȼ�յĻ�ѧ����ʽ��

��֪�������£����ʼס��������Ҿ�Ϊ��ɫ���壬��F��Ϊ��ɫ���嵥�ʣ�A��C��D��E��G��Ϊ��ɫ���壬������A��ֻ��������Ԫ�أ����ǵ�������Ϊ3��1����ش��������⣺
��1��д��A��E�����������ʵĻ�ѧʽ��
A
CH4
CH4
ECO
CO
��CuO
CuO
��2��д��A�ڳ���G����ȫȼ�յĻ�ѧ����ʽ��
CH4+2O2
CO2+2H2O
| ||
CH4+2O2
CO2+2H2O
��
| ||
��������������ṩ����Ϣ����Ͽ�ͼ���з�����B��ͨ�������������D��G����B��ˮ��D��G��������������F�Ǻ�ɫ���嵥�ʣ���F��ͭ�����������Ǻ�ɫ��ĩ����D��Ӧ����ͭ����D��������G����������Ϊ����ͭ��CuO����E���ң�����ͭ����ӦҲ����ͭ��E����ɫ���壬��EΪһ����̼����ѧʽΪCO��һ����̼������ͭ��Ӧ����ͭ�Ͷ�����̼����CΪ������̼����Ϊ̼������A����������ȫȼ�����ɶ�����̼��ˮ���Ƴ�A�к���Ԫ�����࣬������Ԫ�ص�������Ϊ3��1���Ƴ�ԭ�Ӹ����ȣ������Ƴ���ѧʽ��
����⣺B��ͨ�������������D��G����B��ˮ��D��G��������������F�Ǻ�ɫ���嵥�ʣ���F��ͭ�����������Ǻ�ɫ��ĩ����D��Ӧ����ͭ����D��������G����������Ϊ����ͭ��CuO����E���ң�����ͭ����ӦҲ����ͭ��E����ɫ���壬��EΪһ����̼����ѧʽΪCO��һ����̼������ͭ��Ӧ����ͭ�Ͷ�����̼����CΪ������̼����Ϊ̼��A����������ȫȼ�����ɶ�����̼��ˮ����A�к���̼����Ԫ�أ���Ԫ�ص�������Ϊ3��1����̼����ԭ�ӵĸ�����Ϊ
��
=1��4����AΪ���飬��ѧʽΪCH4������ȼ�յĻ�ѧ����ʽΪ��CH4+2O2
CO2+2H2O
�ʴ�Ϊ����1��CH4 CO CuO ��2��CH4+2O2
CO2+2H2O
| 3 |
| 12 |
| 1 |
| 1 |
| ||
�ʴ�Ϊ����1��CH4 CO CuO ��2��CH4+2O2
| ||
����������Ϊ��ͼʽ�����ƶ��⣬��ɴ�����Ŀ���ؼ���������ͻ�ƿڣ�ֱ�ӵó����ۣ�Ȼ������˳���������������м��ƣ���һ�����������ۣ�

��ϰ��ϵ�д�
�����Ŀ
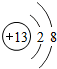 ��2012?�Ӷ���һģ����ͼ��ij���ӵĽṹʾ��ͼ�����жԸ����ӵ��жϲ���ȷ���ǣ�������
��2012?�Ӷ���һģ����ͼ��ij���ӵĽṹʾ��ͼ�����жԸ����ӵ��жϲ���ȷ���ǣ������� ��2012?�Ӷ���һģ�����������������������ڣ�Ӧע��Ӫ�����⣬��ʳ������
��2012?�Ӷ���һģ�����������������������ڣ�Ӧע��Ӫ�����⣬��ʳ������ ��2012?�Ӷ���һģ���Լ�ƿ����1000g�Ȼ�����Һ��ƿ�ϵı�ǩ��ͼ��ʾ���������������ʵ�������
��2012?�Ӷ���һģ���Լ�ƿ����1000g�Ȼ�����Һ��ƿ�ϵı�ǩ��ͼ��ʾ���������������ʵ�������