��Ŀ����
�Ȼ���ͭ��CuCl���ǰ�ɫ��ĩ��������ˮ���Ҵ����۵�422�棬�е�1366�棬�ڿ�����Ѹ�ٱ���������ɫ���������л��ϳɹ�ҵ�еĴ�������ij����ˮ����CaCl2��Na2SO4�����ʣ���Cu��ϡ���ᡢSO2��Ϊԭ�Ϻϳ�CuCl�Ĺ������£�
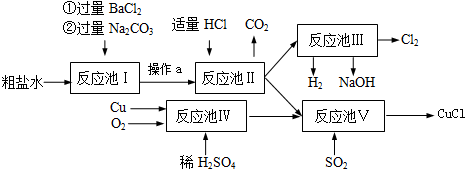
��1�����м�Na2CO3��Һ��������
��2�����з����Ļ�ѧ��Ӧ����ʽΪ
��3�����еķ�Ӧ���ڵ�������½��еģ���Ӧ��Ϊ
��4�����õ���CuCl����������ˮ�Ҵ�ϴ�ӣ�������ո�����ڣ�����2Сʱ����ȴ���ܷ��װ���ò�Ʒ����ո����Ŀ����

��1�����м�Na2CO3��Һ��������
��ȥCa2+������Ba2+
��ȥCa2+������Ba2+
������a������������
����
����2�����з����Ļ�ѧ��Ӧ����ʽΪ
2CuSO4+2NaCl+SO2+2H2O=2CuCl��+2NaHSO4+H2SO4
��2CuSO4+2NaCl+SO2+2H2O=2CuCl��+Na2SO4+2H2SO4
��2CuSO4+2NaCl+SO2+2H2O=2CuCl��+Na2SO4+2H2SO4
2CuSO4+2NaCl+SO2+2H2O=2CuCl��+2NaHSO4+H2SO4
��2CuSO4+2NaCl+SO2+2H2O=2CuCl��+Na2SO4+2H2SO4
��2CuSO4+2NaCl+SO2+2H2O=2CuCl��+Na2SO4+2H2SO4
��3�����еķ�Ӧ���ڵ�������½��еģ���Ӧ��Ϊ
�Ȼ���
�Ȼ���
��ˮ����4�����õ���CuCl����������ˮ�Ҵ�ϴ�ӣ�������ո�����ڣ�����2Сʱ����ȴ���ܷ��װ���ò�Ʒ����ո����Ŀ����
�ӿ��Ҵ���ˮ����������ֹCuCl����������
�ӿ��Ҵ���ˮ����������ֹCuCl����������
����������1��̼������ӿ��Ժͱ������Լ�������֮�䷢����Ӧ����̼����Լ�̼�ᱵ��������Һ���з��벻���Թ������ʵIJ����ǹ��ˣ�
��2���������������֮ͭ����Է���������ԭ��Ӧ�����Ȼ���ͭ������
��3���Ȼ��ƺ�ˮ��������������ƺ���������������
��4���Ҵ���ˮ�ӷ���CuCl���л�ԭ�ԣ����Ա�����������
��2���������������֮ͭ����Է���������ԭ��Ӧ�����Ȼ���ͭ������
��3���Ȼ��ƺ�ˮ��������������ƺ���������������
��4���Ҵ���ˮ�ӷ���CuCl���л�ԭ�ԣ����Ա�����������
����⣺��1����Ӧ���м�Na2CO3��Һ���Դ�����Һ�еij�ȥCa2+�ͳ�ȥ������������ӵĹ�����Ba2+����Һ���з��벻���Թ������ʵIJ����ǹ��ˣ��ʴ�Ϊ����ȥCa2+������Ba2+������
��2���������������֮ͭ����Է���������ԭ��Ӧ�����Ȼ���ͭ��������2CuSO4+2NaCl+SO2+2H2O=2CuCl��+2NaHSO4+H2SO4��2CuSO4+2NaCl+SO2+2H2O=2CuCl��+Na2SO4+2H2SO4���ʴ�Ϊ��2CuSO4+2NaCl+SO2+2H2O=2CuCl��+2NaHSO4+H2SO4��2CuSO4+2NaCl+SO2+2H2O=2CuCl��+Na2SO4+2H2SO4��
��3���Ȼ��ƺ�ˮ��������������ƺ����������������ʴ𰸣��Ȼ���
��4��CuCl����������ˮ�Ҵ�ϴ�ӳ���������ո��������70�����2Сʱ���������Լӿ��Ҵ���ˮ����������ֹCuCl�������������ʴ�Ϊ���ӿ��Ҵ���ˮ����������ֹCuCl������������
��2���������������֮ͭ����Է���������ԭ��Ӧ�����Ȼ���ͭ��������2CuSO4+2NaCl+SO2+2H2O=2CuCl��+2NaHSO4+H2SO4��2CuSO4+2NaCl+SO2+2H2O=2CuCl��+Na2SO4+2H2SO4���ʴ�Ϊ��2CuSO4+2NaCl+SO2+2H2O=2CuCl��+2NaHSO4+H2SO4��2CuSO4+2NaCl+SO2+2H2O=2CuCl��+Na2SO4+2H2SO4��
��3���Ȼ��ƺ�ˮ��������������ƺ����������������ʴ𰸣��Ȼ���
��4��CuCl����������ˮ�Ҵ�ϴ�ӳ���������ո��������70�����2Сʱ���������Լӿ��Ҵ���ˮ����������ֹCuCl�������������ʴ�Ϊ���ӿ��Ҵ���ˮ����������ֹCuCl������������
�����������漰���ʵķ�����ᴿ�ķ����Լ����������صĹ���ԭ���ȷ����֪ʶ��ע��֪ʶ��Ǩ�ƺ�Ӧ���ǽ���Ĺؼ����ѶȲ�̫��

��ϰ��ϵ�д�
 �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
�����Ŀ