��Ŀ����
������Ӣ������ѧ�ң��������������ַ����ⶨ�������ܶȣ�
����һ������ȥˮ�����Ͷ�����̼�Ŀ���ͨ���պ��װ��ͭм�IJ����ܣ�ʹ�����е�����ȫ����ȥ����õ������ܶ�Ϊ1.2572kg/L��
����������������NH3��ͨ�����ȵ�װ������ͭ�IJ����ܣ�����ͭ��������ˮ��������ȥˮ�������õ������ܶ�Ϊ1.2508g/L��
�����βⶨ��״����ͬ�����ʣ�
��1���������з�����Ӧ�Ļ�ѧ����ʽΪ______��
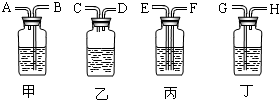
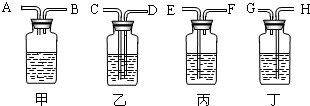
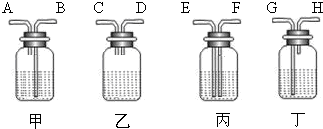
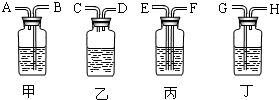
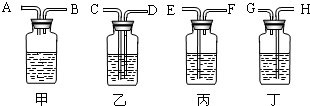
��2������ȥ�����еĶ�����̼���ɹ�ѡ���װ�ã���������˵����ܽ��룬�Ҷ˵������������ͼ��ʾ����ѡ���װ����______��ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ______��
��3����������ַ������ⶨ�ĵ����ܶ���ֵ��ͬ��ԭ��
______
______��
����һ������ȥˮ�����Ͷ�����̼�Ŀ���ͨ���պ��װ��ͭм�IJ����ܣ�ʹ�����е�����ȫ����ȥ����õ������ܶ�Ϊ1.2572kg/L��
����������������NH3��ͨ�����ȵ�װ������ͭ�IJ����ܣ�����ͭ��������ˮ��������ȥˮ�������õ������ܶ�Ϊ1.2508g/L��
�����βⶨ��״����ͬ�����ʣ�
��1���������з�����Ӧ�Ļ�ѧ����ʽΪ______��
��2������ȥ�����еĶ�����̼���ɹ�ѡ���װ�ã���������˵����ܽ��룬�Ҷ˵������������ͼ��ʾ����ѡ���װ����______��ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ______��

��3����������ַ������ⶨ�ĵ����ܶ���ֵ��ͬ��ԭ��
______
______��
��1������������������NH3��ͨ�����ȵ�װ������ͭ�IJ����ܣ�����ͭ��������ˮ��������ѧ����ʽΪ��
2NH3+3CuO
3Cu+3H2O+N2��
��2����ȥ����������ʱ��Ӧʹ�������ͨ����Һ���������̳�������ѡ���װ�ã�������̼�����������Ʒ�Ӧ�����Գ�������̼Ӧͨ������������������Һ�������ķ�ӦΪ��2NaOH+CO2=Na2CO3+H2O��
��3�������ӿ����з���ĵ����к�������ϡ�����壬��ϡ��������ܶȴ��ڵ����������Ǵ����ĵ�����19����ĩ��ǰ�����Ǿ�����Ϊ�����������͵�����ɣ���û�з����������壬�Ӻ��������Ƶõĵ����ܶ��Ǵ��������ܶȣ��ӿ����з���ĵ������ܶ�ʵ���ϰ�����ϡ������������ˣ����Բ�õĵ����ܶ�ƫ��
�ʴ�Ϊ��
��1��2NH3+3CuO
3Cu+3H2O+N2��
��2������2NaOH+CO2=Na2CO3+H2O��
��3������һ�еġ��������к���������ϡ�����壬��ϡ��������ܶȴ��ڵ�����
2NH3+3CuO
| ||
��2����ȥ����������ʱ��Ӧʹ�������ͨ����Һ���������̳�������ѡ���װ�ã�������̼�����������Ʒ�Ӧ�����Գ�������̼Ӧͨ������������������Һ�������ķ�ӦΪ��2NaOH+CO2=Na2CO3+H2O��
��3�������ӿ����з���ĵ����к�������ϡ�����壬��ϡ��������ܶȴ��ڵ����������Ǵ����ĵ�����19����ĩ��ǰ�����Ǿ�����Ϊ�����������͵�����ɣ���û�з����������壬�Ӻ��������Ƶõĵ����ܶ��Ǵ��������ܶȣ��ӿ����з���ĵ������ܶ�ʵ���ϰ�����ϡ������������ˣ����Բ�õĵ����ܶ�ƫ��
�ʴ�Ϊ��
��1��2NH3+3CuO
| ||
��2������2NaOH+CO2=Na2CO3+H2O��
��3������һ�еġ��������к���������ϡ�����壬��ϡ��������ܶȴ��ڵ�����

��ϰ��ϵ�д�
 Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д� ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�
�����Ŀ