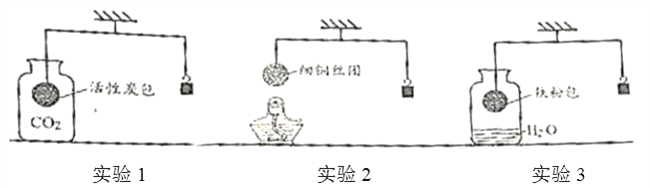
��Ŀ����
����Ŀ��������(��Ҫ�ɷ�ΪFe2O3)����������������
(1)Fe2O3����Ԫ�ص���������Ϊ______��
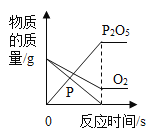
(2)�������ᴿ��õ��Ĵ���Fe2O3����������ij�ֹ�ҵ����(��Ҫ�ɷ�ΪFeO��Fe2O3)���䷴Ӧԭ��Ϊ2Fe2O3��C![]() 4FeO��CO2�����ֽ���̿������Fe2O3��Ͼ��ȣ�������ԭ����ַ�Ӧ����ͼ��ʾΪ��������������淴Ӧʱ��ı仯���ߡ�
4FeO��CO2�����ֽ���̿������Fe2O3��Ͼ��ȣ�������ԭ����ַ�Ӧ����ͼ��ʾΪ��������������淴Ӧʱ��ı仯���ߡ�
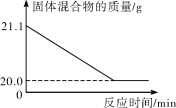
����ͼ��֪������CO2���������Ϊ______g��
�ڼ��㷴Ӧ�����������FeO����������______ (����ݻ�ѧ����ʽд�������ļ��㲽��)��
���𰸡�30%1.1g36%
��������
(1)��Fe2O3�У���Ԫ�ص���������Ϊ![]() ��100%=30%��
��100%=30%��
(2)����ͼ�������غ㶨�ɿɵã�����CO2���������Ϊ 21.1g=-20.0g=1.1g��
���跴Ӧ�����������FeO������Ϊx��
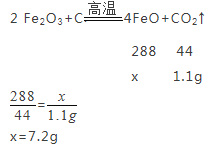
��Ӧ�����������FeO����������Ϊ![]() ��100%=36.0%��
��100%=36.0%��

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д� ����������ϵ�д�
����������ϵ�д�
�����Ŀ