��Ŀ����
�����ǵ�����������Ⱦ����֮һ���ҹ����ϵ���������ĸ߷��أ������PH��5.6��ijУ��ѧ������ȤС���ͬѧ��ȡ�ս������ˮˮ������PH̽ͷ����PH����������ÿ�������Ӳ�һ��PH�������ݼ��±���
| �ⶨʱ�� | 5.05 | 5.10 | 5.15 | 5.20 | 5.25 | 5.30 |
| pH | 4.95 | 4.95 | 4.94 | 4.88 | 4.86 | 4.85 |
�ٸ�����ѧ���й�֪ʶ���Ʋ⡰������ˮ����PH________��
�ڸ����������ݣ��жϸõ���������ˮ�Ƿ�Ϊ���ꣿ�ڲⶨ��ʱ���ڣ���ˮ����������ǿ���Ǽ�����
�۾����飬�õ�����һ�����᳧�����������в���SO2����ʹ�õ�ȼ����Ҫ��ú���Է��������һ�����������Ҫԭ����ʲô��
�������Ĵ�ʩ������������Դ֮�⣬���ɽ����������ķ���________��
��7 ���������ŷ�
�����������к��ж�����̼���壬������̼�ܺ�ˮ��Ӧ����̼�ᣬ̼�������ԣ�����������ˮ�����ԣ�PHӦ��С��7������Һ��PH��7ʱ������PH�ļ�С������ǿ��β�����������ŷſ��Է�ֹ������Ⱦ��
��𣺢�������ˮ��Ϊ�����к��еĶ�����̼��ˮ��Ӧ����̼��������ԣ�PHӦ��С��7�������7��
����Ϊ�����PH��5.6�����Ըõ���������ˮ�����꣮�ڲⶨ��ʱ���ڣ���ˮ��PH��С����������ǿ��
�������һ�����������Ҫԭ���ǣ�úȼ�������ɴ����Ķ���������������Ĺ�����Ҳ�������������������ܺ�ˮ��Ӧ��������������ᣬ��������������γ��������Ҫ���ʣ�
�����Ĵ�ʩ������������Դ֮�⣬���ɽ����������ķ������������ŷţ�������������ŷţ�
�������жϡ�������ˮ����PHʱҪ���ǿ����к��еĶ�����̼��������ʣ�β�����������ŷſ��Լ���Ի�������Ⱦ��
�����������к��ж�����̼���壬������̼�ܺ�ˮ��Ӧ����̼�ᣬ̼�������ԣ�����������ˮ�����ԣ�PHӦ��С��7������Һ��PH��7ʱ������PH�ļ�С������ǿ��β�����������ŷſ��Է�ֹ������Ⱦ��
��𣺢�������ˮ��Ϊ�����к��еĶ�����̼��ˮ��Ӧ����̼��������ԣ�PHӦ��С��7�������7��
����Ϊ�����PH��5.6�����Ըõ���������ˮ�����꣮�ڲⶨ��ʱ���ڣ���ˮ��PH��С����������ǿ��
�������һ�����������Ҫԭ���ǣ�úȼ�������ɴ����Ķ���������������Ĺ�����Ҳ�������������������ܺ�ˮ��Ӧ��������������ᣬ��������������γ��������Ҫ���ʣ�
�����Ĵ�ʩ������������Դ֮�⣬���ɽ����������ķ������������ŷţ�������������ŷţ�
�������жϡ�������ˮ����PHʱҪ���ǿ����к��еĶ�����̼��������ʣ�β�����������ŷſ��Լ���Ի�������Ⱦ��

��ϰ��ϵ�д�
�����Ŀ
 ��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺
��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺ ��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺
��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺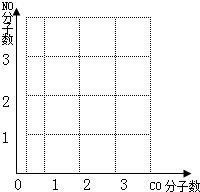

 ��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺
��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺ CO+H2���÷�Ӧ�Ļ��������� ��
CO+H2���÷�Ӧ�Ļ��������� ��