��Ŀ����
��Լ��ˮ������ˮ��Դ��ÿ������Ӧ��������
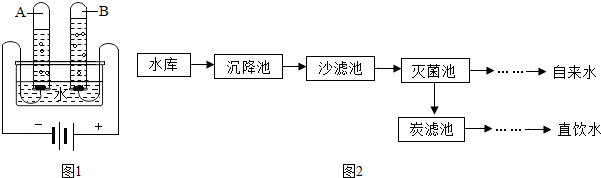
��1����ˮ����ˮ����ˮ������Ȼˮ����Ȼˮ��________ ����������ϡ�����
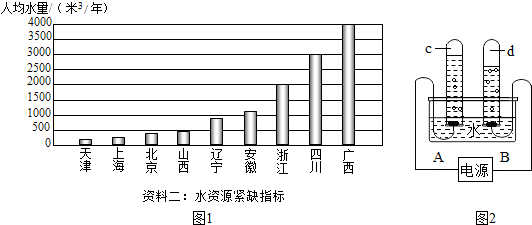
��2������һ���ҹ�����ʡ���˾�ˮ��ͼ
���϶���ˮ��Դ��ȱָ��
| ��ȱ�̶� | ���ȱˮ | �ж�ȱˮ | �ض�ȱˮ | ����ȱˮ |
| �˾�ˮ����m3/���� | 1700��3000 | 1000��1700 | 500��1000 | ��500 |
��3��ˮ���ճ��������кܶ���;���ڹ�ҵ��ˮ����ȡH2����Ҫԭ�ϣ������54gˮ��ȡH2������H2________mol��
�⣺��1��������Ȼˮ���ڳ����Ļ����У���������ܺͲ������Ե����ʣ����ڻ���
��2�����ҹ�����ʡ���˾�ˮ��ͼ��֪���Ϻ����˾�ˮ��С��500m3/�������ڼ���ȱˮ���У�����ÿ���˶�Ӧ�ý�Լ��ˮ�����еĽ�ˮ�취�϶࣬���磺�ڿ��ܵ�������ظ����õȣ�
��3������54gˮ��ȡH2������H2�����ʵ�����X
2H2O 2H2��+O2��
2H2��+O2��
36g 2mol
54g x
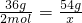 ��ã�X=3mol
��ã�X=3mol
�ʴ�Ϊ����1����ϣ���2������ȱˮ�� �ڿ��ܵ�������ظ����ã���3��3��
��������1��������Ȼˮ�ijɷַ�����
��2�������Ϻ����˾�ˮ����ˮ��Դ��ȱָ������Ϻ��Ľ�ȱ�̶ȣ����ݳ����Ľ�Լ��ˮ�Ĵ�ʩ������
��3�����ݻ�ѧ����ʽ����ˮ������������ɵ����������ʵ�����
������ˮ��Դ�����˵���ѷ��ģ�Ҫ��Լ��ˮ��������Լ��ˮ������������
��2�����ҹ�����ʡ���˾�ˮ��ͼ��֪���Ϻ����˾�ˮ��С��500m3/�������ڼ���ȱˮ���У�����ÿ���˶�Ӧ�ý�Լ��ˮ�����еĽ�ˮ�취�϶࣬���磺�ڿ��ܵ�������ظ����õȣ�
��3������54gˮ��ȡH2������H2�����ʵ�����X
2H2O
 2H2��+O2��
2H2��+O2��36g 2mol
54g x
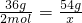 ��ã�X=3mol
��ã�X=3mol�ʴ�Ϊ����1����ϣ���2������ȱˮ�� �ڿ��ܵ�������ظ����ã���3��3��
��������1��������Ȼˮ�ijɷַ�����
��2�������Ϻ����˾�ˮ����ˮ��Դ��ȱָ������Ϻ��Ľ�ȱ�̶ȣ����ݳ����Ľ�Լ��ˮ�Ĵ�ʩ������
��3�����ݻ�ѧ����ʽ����ˮ������������ɵ����������ʵ�����
������ˮ��Դ�����˵���ѷ��ģ�Ҫ��Լ��ˮ��������Լ��ˮ������������

��ϰ��ϵ�д�
�����Ŀ
 ��2012?��������ģ��ˮ��������Ҫ����Դ�������ᳫ��Լ��ˮ������ˮ��Դ��
��2012?��������ģ��ˮ��������Ҫ����Դ�������ᳫ��Լ��ˮ������ˮ��Դ��