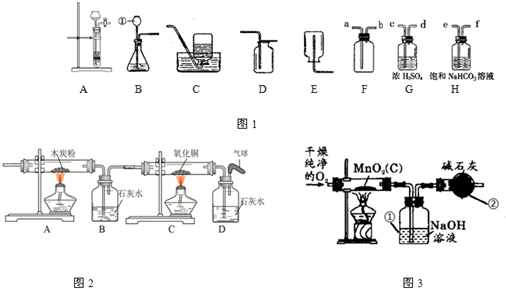
��Ŀ����
ij����������Ʒ�к�������̿��Ϊ�ⶨ����Ʒ�ж������̵�����������ij��ȤС�����������ʵ�鷽����������ͼʵ��װ�ã���һ��������Ʒ��ͨ����﴿����������ʹ����̿�ڼ��������·�Ӧ����CO2�����з����ⶨ��

��1�������ٵ��¶˲���Һ������Ϊ�˷�ֹ______�������ʵ��ѡ���װ������ȡ��������Ӧ�Ļ�ѧ����ʽΪ______��
��2������װ�ÿ������ռ��������壮������ƿ��װ��Ũ�����������������Ӧ��______��ͨ�루ѡ�a����b���������������в�����Ũ����������______��
A��H2B��NH3C��CO2D��O2
��3�������ø��﴿����O2����Ʒ��Ӧ���ⶨ������������������װ�ã���������װ�м�ʯ�ң���������______��
��4��Ϊ��֤װ�ñ��Т��ѽ�CO2������ȫ�����ڢ����֮����붡װ�ý���֤������װ���м�����Լ�Ϊ��______��
A��Ũ���ᡡ B������ʯ��ˮ���� C���ռ���Һ���� D��Ũ����
��5����ȡ8 g����������Ʒ����ʵ�飬����װ�âڷ�Ӧǰ���������Ϊ1.1 g��������Ʒ�ж������̵���������Ϊ���٣�
�⣺��1��Ϊ��ֹ����ӳ���©���ݳ�������©��Ӧ����Һ�����£���װ�������ڹ���������Һ�巴Ӧ��ȡ���壬���ѡ���װ����ȡ����ʱ��Ӧѡ�����������ֽ�ķ�Ӧ��
�ʴ�Ϊ������������ӳ���©����ɢ�������У�2H2O2 2H2O+O2����
2H2O+O2����
��2��װ���ڼ���Ũ�������ڸ�������ʱ������Ӧͨ��װ���ڵ�Ũ���ᣬ��ˣ�����Ӧ��b��ͨ�룻���ڰ����������ᷢ����Ӧ����˰���������Ũ������и��
��ѡB��B��
��3����װ�����������������շ�Ӧ�������Ķ�����̼���壬���ֱ������������������������տ����еĶ�����̼��ʹ�ⶨ������ֽϴ�ƫ�������������ۣ���ֹ�����ж�����̼������������Һ������Ӧ��
�ʴ�Ϊ�����տ����еĶ�����̼��ʹʵ�����ݸ�ȷ��
��4�����������̼���壬Ӧʹ�ó���ʯ��ˮ������ڶ�װ����Ӧ�������ʯ��ˮ��
��ѡB��
��5������Ʒ�ж������̵�����Ϊx
C+O2 CO2
CO2
12 44
x 1.1g
12��44=x��1.1g
��֮�� x=0.3g
��Ʒ�ж������̵���������= =96.25%
=96.25%
����Ʒ�ж������̵���������Ϊ96.25%������ʹ�1�֣�
��������1�����ͷ���װ���г���©������Һ�����µ�ԭ���ж�ʹ�ø�װ����ȡ����ʱ�ķ�Ӧ�Ļ�ѧ����ʽ��
��2������װ�õ�ʹ�÷�������������Ũ�������������ѡ��
��3������ʵ��Ŀ�ģ�����װ����ָ�����������ã�
��4������ʵ��Ŀ�ģ�����װ����Ӧ��ʢ�ŵ�ҩƷ��
��5�����û�ѧ����ʽ���������������������μӷ�Ӧ�����ʵ�������
������ʵ������ȡ����ķ���װ�ÿɷ�Ϊ�������ڹ�����Һ�岻��Ҫ�����ͺͶԹ��壨�����������������֣�
�ʴ�Ϊ������������ӳ���©����ɢ�������У�2H2O2
 2H2O+O2����
2H2O+O2������2��װ���ڼ���Ũ�������ڸ�������ʱ������Ӧͨ��װ���ڵ�Ũ���ᣬ��ˣ�����Ӧ��b��ͨ�룻���ڰ����������ᷢ����Ӧ����˰���������Ũ������и��
��ѡB��B��
��3����װ�����������������շ�Ӧ�������Ķ�����̼���壬���ֱ������������������������տ����еĶ�����̼��ʹ�ⶨ������ֽϴ�ƫ�������������ۣ���ֹ�����ж�����̼������������Һ������Ӧ��
�ʴ�Ϊ�����տ����еĶ�����̼��ʹʵ�����ݸ�ȷ��
��4�����������̼���壬Ӧʹ�ó���ʯ��ˮ������ڶ�װ����Ӧ�������ʯ��ˮ��
��ѡB��
��5������Ʒ�ж������̵�����Ϊx
C+O2
 CO2
CO212 44
x 1.1g
12��44=x��1.1g
��֮�� x=0.3g
��Ʒ�ж������̵���������=
 =96.25%
=96.25%����Ʒ�ж������̵���������Ϊ96.25%������ʹ�1�֣�
��������1�����ͷ���װ���г���©������Һ�����µ�ԭ���ж�ʹ�ø�װ����ȡ����ʱ�ķ�Ӧ�Ļ�ѧ����ʽ��
��2������װ�õ�ʹ�÷�������������Ũ�������������ѡ��
��3������ʵ��Ŀ�ģ�����װ����ָ�����������ã�
��4������ʵ��Ŀ�ģ�����װ����Ӧ��ʢ�ŵ�ҩƷ��
��5�����û�ѧ����ʽ���������������������μӷ�Ӧ�����ʵ�������
������ʵ������ȡ����ķ���װ�ÿɷ�Ϊ�������ڹ�����Һ�岻��Ҫ�����ͺͶԹ��壨�����������������֣�

��ϰ��ϵ�д�
�����Ŀ