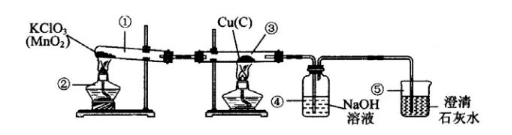
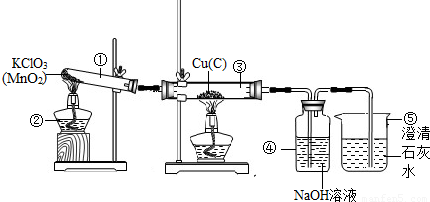
��Ŀ����
ij�Ƽ�С���ͬѧ������Ȼ��Դ����˺��ɫ��ͭ�ۣ�������̿����Ϊ�˲ⶨ��ͭ����Ʒ��ͭ�������������ٷֺ�������ȡW gͭ����Ʒ���������ʵ��װ�ã�
��1�������ڡ��ݵ������ǣ���
��2���١����з�����Ӧ�Ļ�ѧ����ʽΪ����
��3��װ�â��е�ʵ��������
��4������ʵ��ʱ����Ϩ�������ƾ��ƣ�����ȴ�����п��ܵ��µĺ����
��5����������װ�ã�ͨ��������Ӧǰ��װ�âܵ��������õ�CO2���������������ͭ������������ʵ������к���ˮ������Ӱ�죩��Ϊ��ȷ����õ�CO2����ȷ�ɿ����ڱ�֤װ�ò�©��������ȷ�������淶��ǰ���£�����Ϊ����Ҫ��������
����������Ҫ��ȷ��ʵ���Ŀ���ǡ��ⶨ��ͭ����Ʒ��ͭ��������������Ȼ��Χ�Ƹ�ʵ��Ŀ�ģ��������ʵ��װ���п����漰�ķ�Ӧԭ����װ�����ã�װ�â�������KClO3���ȴ��ֽ���ȡ������װ�â�������������ͭ����Ʒ�е�Cu��C������Ӧ�ֱ�����CuO��ɫ�����CO2���壻���ɵ�CO2�������װ�â�����NaOH��Һ��Ӧ�������գ��ɸ���װ�âܵ����ز������̿���ɵ�CO2���������ٻ���Ϊ����̿����������ͭ����Ʒ������ȥ̿����������ͭ���������ɴ����ͭ������������װ�â��еij���ʯ��ˮ�ɼ����Ƿ���δ��NaOH��Һ���յ�CO2ͨ��������������ش�1������5�����й����⣮
����⣺��1��������Ϊ���ڼ��ȵľƾ��ƣ�����������ʢ�Žϴ���Һ����ܽ�����Ҫ���ձ���
�ʴ�Ϊ���ƾ��ƣ��ձ���
��2����Ӧ��Ϊ������ڼ��ȺͶ������̴������£��ֽ������Ȼ��غ���������Ӧ��Ϊ����������Һ�������������̼������̼���ƺ�ˮ��
�ʴ�Ϊ��2KClO3
2KCl+3O2����2NaOH+CO2=Na2CO3+H2O��
��3��װ�â��к��ɫ�����ڼ���������������������Ӧ���γɺ�ɫ������ͭ������������̼���ڼ�����������������Ӧ�γ���ɫ���������̼��
�ʴ�Ϊ�����ɫ��ͭ�۱�ɺ�ɫ��
��4��ʵ�����ʱ�������ֹͣ���ȣ�װ���������������������С���������ձ������ƿ��Һ�嵹�����Թܼ������ܣ���ʹ�Թܼ�����ͻȻ�����ը�ѣ�
�ʴ�Ϊ����Һ�������������ܺ��Թ����ѣ�
��5���������⡰ͨ��������Ӧǰ��װ�âܵ��������õ�CO2���������������ͭ��������������ԭ���͡�ȷ����õ�CO2����ȷ�ɿ�����ʵ��Ҫ���ڱ�֤װ�ò�©��������ȷ�������淶��ǰ���¡���������Ҫ����������һ������ر���������������������ṩ����������������̿������CO2������NaOH��ҺҲ�����������Ա�֤�����ɵ�CO2ȫ�����գ�
�ʴ�Ϊ������ر������������������NaOH��ҺҲ����������
�ʴ�Ϊ���ƾ��ƣ��ձ���
��2����Ӧ��Ϊ������ڼ��ȺͶ������̴������£��ֽ������Ȼ��غ���������Ӧ��Ϊ����������Һ�������������̼������̼���ƺ�ˮ��
�ʴ�Ϊ��2KClO3
| ||
| �� |
��3��װ�â��к��ɫ�����ڼ���������������������Ӧ���γɺ�ɫ������ͭ������������̼���ڼ�����������������Ӧ�γ���ɫ���������̼��
�ʴ�Ϊ�����ɫ��ͭ�۱�ɺ�ɫ��
��4��ʵ�����ʱ�������ֹͣ���ȣ�װ���������������������С���������ձ������ƿ��Һ�嵹�����Թܼ������ܣ���ʹ�Թܼ�����ͻȻ�����ը�ѣ�
�ʴ�Ϊ����Һ�������������ܺ��Թ����ѣ�
��5���������⡰ͨ��������Ӧǰ��װ�âܵ��������õ�CO2���������������ͭ��������������ԭ���͡�ȷ����õ�CO2����ȷ�ɿ�����ʵ��Ҫ���ڱ�֤װ�ò�©��������ȷ�������淶��ǰ���¡���������Ҫ����������һ������ر���������������������ṩ����������������̿������CO2������NaOH��ҺҲ�����������Ա�֤�����ɵ�CO2ȫ�����գ�
�ʴ�Ϊ������ر������������������NaOH��ҺҲ����������
�����������ۺ�̽��ʵ���⣬�ɰ����ۺ�̽��ʵ����������衱��˼·չ������������Ҫ��ȷ��ʵ���Ŀ�ģ�Ȼ��Χ�Ƹ�ʵ��Ŀ�ģ��������ʵ��װ���п����漰�ķ�Ӧԭ����װ�����ã�����������ش��йص����⣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ