��Ŀ����
ij��ѧ�С�����ô�����ȡ�ռ��������ʵ�飺
��ȡ20g������Ʒ(�������ʲ�����ˮ��Ҳ�����������ʷ�Ӧ)�������м���34.1gˮʹ����ȫ�ܽ⣬Ȼ���ټ���100g��������������Ϊl7.1%������������Һ����ǡ����ȫ��Ӧ�����˵õ�������һ�ֲ�������Һ����ش����⣺
(1)����ʵ�鲽���У���ָ�������еĴ��� ��
(2)��д��������Ӧ�Ļ�ѧ����ʽ ��
(3)�г��μӷ�Ӧ������������(x)�ı���ʽΪ ��
(4)����Ʒ�Ĵ���Ϊ ��
(5)��Ӧ��������Һ��������������Ϊ ��
��1������ʱû�ò��������� ��2��Na2CO3+ Ba(OH)2==BaCO3��+2NaOH
��3�� ��4��53% ��5��6.4%
��4��53% ��5��6.4%
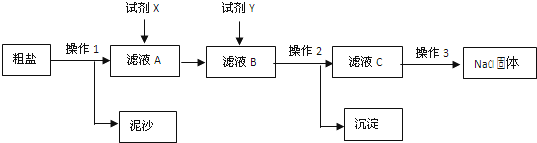
�������������(1)����ʱ����������������������3�����ݻ�ѧ����ʽ�ļ��㣬����ʽΪ ����4�����ݣ�2��������μӷ�Ӧ��̼���Ƶ�������Ϊ20g������Ʒ�д�����̼���Ƶ���������5��������Һ���������Ķ��壬������������������Һ������Ȼ����ٷ�֮�٣�����������Ϊ��Ӧ���ɵ��������Ƶ����������ݣ�2���л�ѧ����ʽ�����������Һ������Ϊ20g������Ʒ�д�����̼���Ƶ���������34.1gˮ���ټ���100g��������������Ϊl7.1%������������Һ������������ȥ���ɵ�̼�ᱵ�����������ݣ�2���л�ѧ����ʽ�����������
����4�����ݣ�2��������μӷ�Ӧ��̼���Ƶ�������Ϊ20g������Ʒ�д�����̼���Ƶ���������5��������Һ���������Ķ��壬������������������Һ������Ȼ����ٷ�֮�٣�����������Ϊ��Ӧ���ɵ��������Ƶ����������ݣ�2���л�ѧ����ʽ�����������Һ������Ϊ20g������Ʒ�д�����̼���Ƶ���������34.1gˮ���ټ���100g��������������Ϊl7.1%������������Һ������������ȥ���ɵ�̼�ᱵ�����������ݣ�2���л�ѧ����ʽ�����������
���㣺��ѧ����ʽ�ļ���
������������ĿҪ�в�ε�������Ҫ���꣬�����п��ؿ����ͣ�ע�����ϸ�ġ�