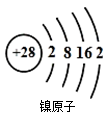
��Ŀ����
012��6�£���������������DZˮ���������ҹ�������DZ�¼�¼��ʵ�����ҹ��������չ���ش��Խ��
��1���������������ܿ�����ѹ���ѺϽ����ס����ѺϽ����� ���ϣ�����Ӳ��(�С�ڡ����ڡ�)�����ѵ�Ӳ�ȣ�
��2����DZʱ�����˲������������������ͬ�������������������ԼΪ %��
��3���DZ���пɹ۲캣�ġ���Һ���롰��Ȫ������ǰ���Ǻ�ˮ������£������Ҽ��Ⱥ��ط����Ļ���ú�ˮ�еķḻ������ط��ر�ʱ������ˮ���������������Ҫԭ���ǣ� �����ߵ��γ�ԭ��֮һ�Ǻ�����Ȼ�����������Ȼ������Ҫ�ɷ��Ǽ��飬��ȫȼ�����ɶ�����̼��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��1���������������ܿ�����ѹ���ѺϽ����ס����ѺϽ����� ���ϣ�����Ӳ��(�С�ڡ����ڡ�)�����ѵ�Ӳ�ȣ�
��2����DZʱ�����˲������������������ͬ�������������������ԼΪ %��
��3���DZ���пɹ۲캣�ġ���Һ���롰��Ȫ������ǰ���Ǻ�ˮ������£������Ҽ��Ⱥ��ط����Ļ���ú�ˮ�еķḻ������ط��ر�ʱ������ˮ���������������Ҫԭ���ǣ� �����ߵ��γ�ԭ��֮һ�Ǻ�����Ȼ�����������Ȼ������Ҫ�ɷ��Ǽ��飬��ȫȼ�����ɶ�����̼��ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��1������ ���ڣ� ��2��21��
��3���¶Ƚ��ͣ�������ܽ�ȼ�С���ᾧ���� CH4+2O2��ȼ2H2O+CO2��
��3���¶Ƚ��ͣ�������ܽ�ȼ�С���ᾧ���� CH4+2O2��ȼ2H2O+CO2��
�����������1���ѺϽ��ǽ����ѵĺϽ����ڽ������ϣ��Ͻ��Ӳ��Ҫ���ڴ����Ľ�����Ӳ�ȡ�
��2���������������������Ϊ21%
��3�������ʵ��ܽ�����¶ȵĽ��Ͷ���С�����¶Ƚϸߵĺ�ˮ����ˮʱ���¶Ƚ��ͣ������ܽ�Ŀ����ʵ��ܽ�Ƚ��ͣ�ԭ���ܽ�Ŀ�������ȫ���ܽ��������
������ȫȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪCH4+2O2��ȼ 2H2O+CO2
�����������ϡ��������ſ����˽��������ʡ������ijɷ֡��ܽ�ȵ�֪ʶ�㣬��ͨ����Ӧ֪ʶ���н��

��ϰ��ϵ�д�
�����Ŀ
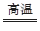 2Fe��3CO2
2Fe��3CO2

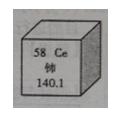
 2Fe+3C02���÷�Ӧ��������C0���� �ԣ�
2Fe+3C02���÷�Ӧ��������C0���� �ԣ�