��Ŀ����
 ��ͼ��һ�ּӸ�ʳ�ΰ�װ��ǩ�ϵIJ�������˵��������ϸ�Ķ���ش��������⣺
��ͼ��һ�ּӸ�ʳ�ΰ�װ��ǩ�ϵIJ�������˵��������ϸ�Ķ���ش��������⣺
��1����ǩ�ϵġ��ơ���ָ________��
A��̼��ơ���B�����ʸơ���C����Ԫ��
��2��Ϊ�˼���������Ƿ���̼��ƣ��ڼ�ͥ�������ѡ�õ�������________��
��3��Ϊ�˲ⶨ�˼Ӹ�ʳ����̼��Ƶĺ�����ȡ10g����������ˮ�������������ᣬ����0.132g������̼���÷�Ӧ�Ļ�ѧ����ʽΪ________���˼Ӹ�ʳ���У�̼��Ƶ���������Ϊ________��
��4�����˼Ӹ�ʳ���к��������Ϊ12.7mg�����е���أ�KIO3��������________mg��
�⣺��1����Ϊij�ּӸ�ʳ���еĸ����Ի��������ʽ���ڣ�����������Ԫ����ɵģ��ʰ�װ��ǩ�ϸƺ�����ָ��Ԫ�أ���ѡC
��2����Ϊ̼����к���̼������ӣ�����ˮ�����ᷴӦ���������ɣ����ڼ�ͥ�������ѡ��ʳ��ˮ�����������Ƿ���̼��ƣ��ʴ�Ϊ������
��3������뷴Ӧ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 0.132g
��100��44=x��0.132g��
��֮�ã�x=0.3g��
�˼Ӹ�ʳ����̼��Ƶ���������= ��100%=3%��
��100%=3%��
��4�����е���أ�KIO3��������Ϊ��12.7mg��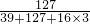 �T21.4mg
�T21.4mg
�ʴ�Ϊ��
��3��CaCO3+2HCl�TCaCl2+CO2��+H2O 3%
��4��21.4
��������1�����ݰ�װ�ṩ����Ԫ����̼����ṩ��̼����ǻ��������������Ԫ����ɵģ��ʰ�װ��ǩ�ϸƺ�����ָ��Ԫ�أ�
��2����Ϊ̼����к���̼������ӣ�����ˮ�����ᷴӦ���������ɣ��ʼ���������Ƿ���̼��ƣ��ڼ�ͥ�������ѡ�õ�����Ӧ���������ʻ�ˮ��
��3������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ɵĶ�����̼�����������ɼ�������뷴Ӧ��̼��Ƶ���������10g��������̼��Ƶ���������Ȼ���ټ�����˼Ӹ�ʳ���У�̼��Ƶ�����������
��4�����ݵ�Ԫ�ص���������õ���أ�KIO3����������
������������Ҫ����ѧ�����û�ѧʽ��Ԫ�ص�����������ʽ�Լ���ѧ����ʽ���м����������
��2����Ϊ̼����к���̼������ӣ�����ˮ�����ᷴӦ���������ɣ����ڼ�ͥ�������ѡ��ʳ��ˮ�����������Ƿ���̼��ƣ��ʴ�Ϊ������
��3������뷴Ӧ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 0.132g
��100��44=x��0.132g��
��֮�ã�x=0.3g��
�˼Ӹ�ʳ����̼��Ƶ���������=
 ��100%=3%��
��100%=3%����4�����е���أ�KIO3��������Ϊ��12.7mg��
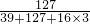 �T21.4mg
�T21.4mg�ʴ�Ϊ��
��3��CaCO3+2HCl�TCaCl2+CO2��+H2O 3%
��4��21.4
��������1�����ݰ�װ�ṩ����Ԫ����̼����ṩ��̼����ǻ��������������Ԫ����ɵģ��ʰ�װ��ǩ�ϸƺ�����ָ��Ԫ�أ�
��2����Ϊ̼����к���̼������ӣ�����ˮ�����ᷴӦ���������ɣ��ʼ���������Ƿ���̼��ƣ��ڼ�ͥ�������ѡ�õ�����Ӧ���������ʻ�ˮ��
��3������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ɵĶ�����̼�����������ɼ�������뷴Ӧ��̼��Ƶ���������10g��������̼��Ƶ���������Ȼ���ټ�����˼Ӹ�ʳ���У�̼��Ƶ�����������
��4�����ݵ�Ԫ�ص���������õ���أ�KIO3����������
������������Ҫ����ѧ�����û�ѧʽ��Ԫ�ص�����������ʽ�Լ���ѧ����ʽ���м����������

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
�����Ŀ
����ͼ����ȷ��ӳ���ж�Ӧ�仯��ϵ����
| ��� | ʵ������ | x�Ậ�� | y�Ậ�� | ����ͼ |
| �� | ��һ�������ۺ�ͭ���в��ϵμ�����ͭ��Һ | ����ͭ��Һ���� | ����ͭ ������ |  |
| �� | ������������Һ����μ���ϡ���� | ϡ�������� | ������Һ ��ˮ������ | |
| �� | һ���¶��£���ij�������Ȼ�����Һ�м����Ȼ��ƹ��� | �����Ȼ������� | ��Һ������ ���������� | |
| �� | ���ȸ��������ȡ���� | ����ʱ�� | ʣ������� ��Ԫ������ |
- A.�٢�
- B.�ۢ�
- C.�٢�
- D.�ڢ�
 ͨ��ѧϰ��ͬѧ��Ӧ��֪���ж���;��������ȡ�������磺
ͨ��ѧϰ��ͬѧ��Ӧ��֪���ж���;��������ȡ�������磺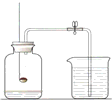 ���������������IJⶨ���ش��������⣺
���������������IJⶨ���ش��������⣺ CH3OH����X�Ļ�ѧʽΪ________��
CH3OH����X�Ļ�ѧʽΪ________��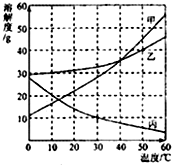 �ס��ҡ����������ʵ��ܽ��������ͼ��ʾ������ͼ����Ϣ�ش��������⣺
�ס��ҡ����������ʵ��ܽ��������ͼ��ʾ������ͼ����Ϣ�ش��������⣺