��Ŀ����
����Ŀ��ij�οɳ���ʳ��(�׳ƾ���)�к���CaCl2 �� Ϊ�ⶨCaCl2�ĺ�����ȷ������������22.8g�������м���87.2��ˮʹ����ȫ�ܽ⣬Ȼ�������м���10.6%��Na2CO3��Һ�����ɳ��������������Na2CO3��Һ��������ϵ��ͼ��ʾ��ǡ����ȫ��Ӧʱ����ʳ����Һ��Ϊ��������Һ����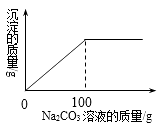
��
��1��������CaCl2������������
��2��ǡ����ȫ��Ӧ��������Һ��NaCl����������(���������һλС��)��
���𰸡�
��1������辮����CaCl2������Ϊxg,��Ӧ�����Ȼ�������Ϊyg,���ɳ���zg
Na2CO3 �� | CaCl2 �� | CaCO3���� | 2NaCl |
106 | 71 | 100 | 117 |
10.6%��100g | x | z | y |
![]() ��
�� ![]() ,x��7.1g. ������CaCl2������������
,x��7.1g. ������CaCl2������������ ![]() ��100����48.7%��
��100����48.7%��
![]() ��
�� ![]() ,y��11.7g.
,y��11.7g. ![]() ��
�� ![]() ,z��10g
,z��10g
��þ�����CaCl2������������48.7%
��2�����ǡ����ȫ��Ӧ��������Һ��NaCl������������ ![]() ��100����11.7%.
��100����11.7%.
���ǡ����ȫ��Ӧ��������Һ��NaCl������������11.7%
��������������Ҫ���������غ㶨�ɵ�Ӧ���Լ����ݻ�ѧ����ʽ���м��㡣���÷���ʽ����ʱ������Ҫ��ȷ��д��ѧ����ʽ���ٸ��ݷ���ʽ�ҳ��漰�����ʵ�������ϵ�����������������ʵ����������Ƚ��ע����뷽��ʽ�����������Dzμӷ�Ӧ�����ɵĴ����������������Һ��ķ�Ӧ����μӷ�Ӧ������Ϊ��Һ�е����ʡ�
�����㾫����������Ҫ�����˸��ݻ�ѧ��Ӧ����ʽ�ļ�������֪ʶ�㣬��Ҫ���ո����ʼ�������=ϵ������Է�������֮�Ȳ�����ȷ�����⣮