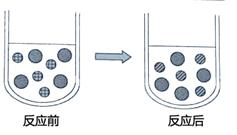
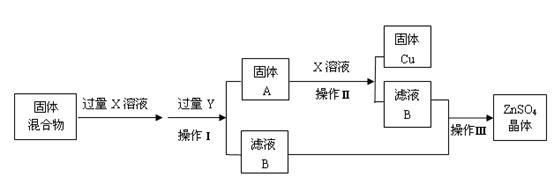
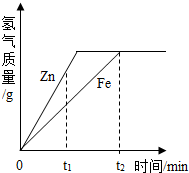
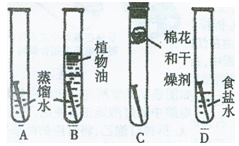
��Ŀ����
��5�֣���������������벻���������ϣ����ſƼ�ˮƽ�IJ�����ߣ���������Ͻ����ճ������а�����Խ��Խ��Ҫ�Ľ�ɫ��
��1��ͭҲ�����⣬ͭ�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ��Cu2��OH��2CO3������ͭ������е�������ˮ���� ����ͬ���õĽ����
��2����ҵ����CO��ԭ��Fe3O4�Ĵ�����ʯұ���������Ļ�ѧ����Ϊ ��
��3��ұ��2000t������3%����������Ҫ��Fe3O490%�Ĵ�����ʯ������ = �������ʽ����
��1��ͭҲ�����⣬ͭ�����Ҫ�ɷ��Ǽ�ʽ̼��ͭ��Cu2��OH��2CO3������ͭ������е�������ˮ���� ����ͬ���õĽ����
��2����ҵ����CO��ԭ��Fe3O4�Ĵ�����ʯұ���������Ļ�ѧ����Ϊ ��
��3��ұ��2000t������3%����������Ҫ��Fe3O490%�Ĵ�����ʯ������ = �������ʽ����
��1��CO2��1�֣�����2��4CO+ Fe3O4���� 3Fe+4CO2��2�֣�δ��ƽ��©д������1�֣���
��3��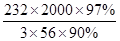 ��2�֣�Լ���ĺ����𰸼�2976.7tҲ���֣�
��2�֣�Լ���ĺ����𰸼�2976.7tҲ���֣�
��3��
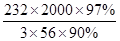 ��2�֣�Լ���ĺ����𰸼�2976.7tҲ���֣�
��2�֣�Լ���ĺ����𰸼�2976.7tҲ���֣������������1����ѧ��Ӧǰ��Ԫ������䣬��ʽ̼��ͭ�к���̼Ԫ�أ���ͭ����ʱһ���к���̼Ԫ�ص����ʲμӣ������к�̼Ԫ�ص������Ƕ�����̼������ͭ������ͭ��������ˮ��������̼��ͬ���õĽ������2����ҵ����һ����̼��ԭFe3O4��������Ҫ������CO�Ļ�ԭ�ԣ��ڸ����º�Fe3O4��Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��4CO+Fe3O4����3Fe+4CO2��
��3������Ҫ��Fe3O490%�Ĵ�����ʯ������Ϊx
4CO+Fe3O4����3Fe+4CO2
232 168
90%x 2000t����1-3%��
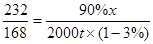

��ϰ��ϵ�д�
�����Ŀ