��Ŀ����
��ҵ�����г�����NaCl��Na2SO4�����ʣ�������ͼװ�òⶨ��ҵ��������Ч�ɷֵĺ�����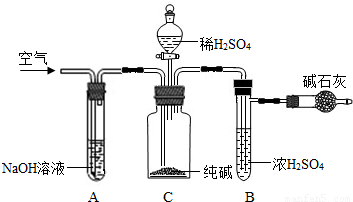
ʵ����̵���Ҫ�����ǣ�
��ȷ��ȡ��������x g��x��2����������ƿC�У���ȷ����װ�м�ʯ�ң�������CO2���ĸ���ܵ�����y g���۴ӷ�Һ©���л���ע��ϡH2SO4�������ٲ�������Ϊֹ���ܻ���������������ӣ�Ȼ�����ж�£�ȷ����������z g��
���������ʵ�飬�ش��������⣺
��1��װ��C�з�����Ӧ�Ļ�ѧ����ʽΪ______��
��2��װ��A��������______���������װ��A���ᵼ��ʵ����ƫ______�������С�����䡱����ͬ����
��3��װ��B��������______���������װ��B���ᵼ��ʵ����ƫ______��
��4���ڢܲ��л���ͨ�������������______�������ͨ��������ᵼ��ʵ����ƫ______��
��5��������Na2CO3�����������ļ���ʽΪ______��
��6������26.5gNa2CO3��NaCl�Ļ��������м���������������Ϊ10%������109.5g����ַ�Ӧ���ټ����ܶ�Ϊ1g/cm3��������������Ϊ10%������������Һ40mL����ʱǡ����ȫ��Ӧ��������Һ��pHΪ7������
��ԭ�������̼���Ƶ�����������
��������Һ�����ʵ�����������
���𰸡���������1������д����ʽ�IJ����ע������ش��⣻
��2�����ڿ����к��ж�����̼������������������Һ�����տ����ж�����̼��
��3������Ũ������ص㿼�ǣ�
��4�����ڷ�Ӧ���˹��ƿ�д����ж�����̼������һ�����Ŀ�������Ϊ���������ǵģ�
��5�����ݼ�ʯ�ң�������CO2���ĸ���ܵ��������������������ɵĶ�����̼���������ٸ��ݶ�����̼���������̼���Ƶ��������ٳ�����Ʒ�����������ɣ�
��6�������������Ƶ�����������������Ʒ�Ӧ���ĵ��������Ȼ�������������ɵ��Ȼ��Ƶ��������������ܵ��Ȼ����������ȥ���������Ʒ�Ӧ���Ȼ����������������̼���Ʒ�Ӧ���Ȼ���������������ø��������̼���Ƶ������������ɵ��Ȼ��ƺͶ�����̼������������̼������������26.5g���̼���ƺ���������������������Ȼ��Ƽ��������������������ٳ�������Һ���������ٷ�֮�٣�����Һ���������˼�ȥ������̼��������
����⣺��1����Ӧ����̼���ƺ����ᣬ�������������ơ�ˮ��������̼�����ù۲취��ƽ���ɣ�������̼��������������ţ�
��2��������������Һ�����տ����ж�����̼��ֹ������̼�����ʯ����Ӱ��ʵ��Ч����
��3��Ũ���������ˮ���������������������̼�ģ��������Ũ���ᣬ�ͻὫ������̼�е�ˮ������Ϊ�Ƕ�����̼���ᵼ�¶�����̼������ƫ���������̼��������Ҳƫ�ʽ��ƫ��
��4�����ڷ�Ӧ���˹��ƿ�д����ж�����̼������һ�����Ŀ������ǽ������Ķ�����̼��ȫ�����ʯ���У�
��5������Ҫ̼���Ƶ�����ΪR
��Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 44
R z-y
�б���ʽ��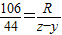
���R=
����������Na2CO3�����������ļ���ʽΪ ×100%��
×100%��
��6���⣺����NaOH��Һ��Ӧ�������������Ϊx��ͬʱ����NaCl������Ϊy1��
NaOH+HCl=NaCl+H2O
40 36.5 58.5
40mL×1g/mL×10% x×10% y1
 =
= ��x=36.5g
��x=36.5g
 =
= ��y1=5.85g
��y1=5.85g
��μӷ�Ӧ��̼���Ƶ�����ΪM���÷�Ӧ���ɵ��Ȼ��Ƶ�����Ϊy2�����ɶ�����̼������ΪZ��
Na2CO3 +2HCl�T2NaCl+CO2��+H2O
106 73 117 44
M ��109.5g-36.5g��×10% y2 Z
 =
= ��M=10.6g
��M=10.6g
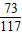 =
=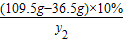 ��y2=11.7g
��y2=11.7g
 =
=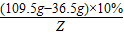 Z=4.4g
Z=4.4g
��1��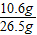 ×100%=40%
×100%=40%
��2�� ×100%=19.5%
×100%=19.5%
��ԭ�������̼���Ƶ���������Ϊ40%��
������Һ�����ʵ�����������19.5%��
�ʴ�Ϊ��
��1��Na2CO3+H2SO4=Na2SO4+CO2��+H2O��
��2����ȥ�����е�CO2 ��
��3������CO2 ��
��4��ʹ���ƿ��������Na2CO3������CO2���ų� С��
��5��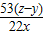 ×100%��
×100%��
��6��40%��19.5%��
��������������׳����ĵط��Ǽ���������������ʱ�������������֣�������Һ����ʱ�����˼�ȥ���ɵĶ�����̼��������
��2�����ڿ����к��ж�����̼������������������Һ�����տ����ж�����̼��
��3������Ũ������ص㿼�ǣ�
��4�����ڷ�Ӧ���˹��ƿ�д����ж�����̼������һ�����Ŀ�������Ϊ���������ǵģ�
��5�����ݼ�ʯ�ң�������CO2���ĸ���ܵ��������������������ɵĶ�����̼���������ٸ��ݶ�����̼���������̼���Ƶ��������ٳ�����Ʒ�����������ɣ�
��6�������������Ƶ�����������������Ʒ�Ӧ���ĵ��������Ȼ�������������ɵ��Ȼ��Ƶ��������������ܵ��Ȼ����������ȥ���������Ʒ�Ӧ���Ȼ����������������̼���Ʒ�Ӧ���Ȼ���������������ø��������̼���Ƶ������������ɵ��Ȼ��ƺͶ�����̼������������̼������������26.5g���̼���ƺ���������������������Ȼ��Ƽ��������������������ٳ�������Һ���������ٷ�֮�٣�����Һ���������˼�ȥ������̼��������
����⣺��1����Ӧ����̼���ƺ����ᣬ�������������ơ�ˮ��������̼�����ù۲취��ƽ���ɣ�������̼��������������ţ�
��2��������������Һ�����տ����ж�����̼��ֹ������̼�����ʯ����Ӱ��ʵ��Ч����
��3��Ũ���������ˮ���������������������̼�ģ��������Ũ���ᣬ�ͻὫ������̼�е�ˮ������Ϊ�Ƕ�����̼���ᵼ�¶�����̼������ƫ���������̼��������Ҳƫ�ʽ��ƫ��
��4�����ڷ�Ӧ���˹��ƿ�д����ж�����̼������һ�����Ŀ������ǽ������Ķ�����̼��ȫ�����ʯ���У�
��5������Ҫ̼���Ƶ�����ΪR
��Na2CO3+H2SO4�TNa2SO4+H2O+CO2��
106 44
R z-y
�б���ʽ��
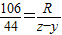
���R=

����������Na2CO3�����������ļ���ʽΪ
 ×100%��
×100%����6���⣺����NaOH��Һ��Ӧ�������������Ϊx��ͬʱ����NaCl������Ϊy1��
NaOH+HCl=NaCl+H2O
40 36.5 58.5
40mL×1g/mL×10% x×10% y1
 =
= ��x=36.5g
��x=36.5g =
= ��y1=5.85g
��y1=5.85g��μӷ�Ӧ��̼���Ƶ�����ΪM���÷�Ӧ���ɵ��Ȼ��Ƶ�����Ϊy2�����ɶ�����̼������ΪZ��
Na2CO3 +2HCl�T2NaCl+CO2��+H2O
106 73 117 44
M ��109.5g-36.5g��×10% y2 Z
 =
= ��M=10.6g
��M=10.6g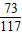 =
=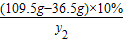 ��y2=11.7g
��y2=11.7g =
=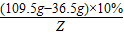 Z=4.4g
Z=4.4g��1��
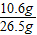 ×100%=40%
×100%=40%��2��
 ×100%=19.5%
×100%=19.5%��ԭ�������̼���Ƶ���������Ϊ40%��
������Һ�����ʵ�����������19.5%��
�ʴ�Ϊ��
��1��Na2CO3+H2SO4=Na2SO4+CO2��+H2O��
��2����ȥ�����е�CO2 ��
��3������CO2 ��
��4��ʹ���ƿ��������Na2CO3������CO2���ų� С��
��5��
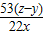 ×100%��
×100%����6��40%��19.5%��
��������������׳����ĵط��Ǽ���������������ʱ�������������֣�������Һ����ʱ�����˼�ȥ���ɵĶ�����̼��������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��ҵ�����г�����ʳ�Σ���Ҫ�ɷ�ΪNa2CO3����ΪNaCl����ij������Ϊ�˲ⶨһ��������Na2CO3���������������������·���ʵ�飺ȡ������Ʒ�����ձ��У���ˮ����ܽ⣬�����μ�ϡ���ᣬ������������Ϊֹ���й��������±���ʾ������Ʒ��Na2CO3������������
| ���� | ��Ʒ���� | ˮ������ | ����ϡ�������� | ��Ӧ����Һ���� |
| ������g�� | 22 | 50 | 56.8 | 120 |
��ҵ�����г�����ʳ�Σ���Ҫ�ɷ�ΪNa2CO3����ΪNaCl����ij������Ϊ�˲ⶨһ��������Na2CO3���������������������·���ʵ�飺ȡ������Ʒ�����ձ��У���ˮ����ܽ⣬�����μ�ϡ���ᣬ������������Ϊֹ���й��������±���ʾ������Ʒ��Na2CO3������������
�������õ������ԭ��������C-12 O-16 Na-23��
| ���� | ��Ʒ���� | ˮ������ | ����ϡ�������� | ��Ӧ����Һ���� |
| ������g�� | 22 | 50 | 56.8 | 120 |